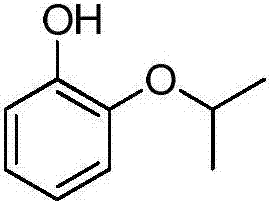
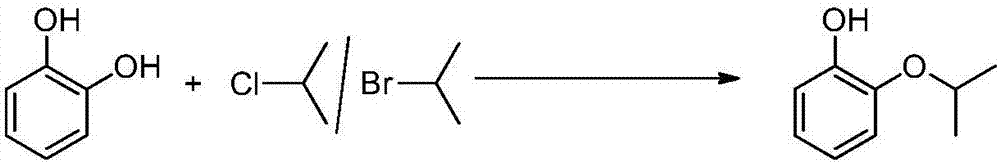
Preparation method of pyrocatechol monoisopropyl ether
A technology of o-isopropoxyphenol and isopropyl, applied in the field of preparation of o-isopropoxyphenol, can solve the problems of low product purity and yield, reduced product purity and yield, large amount of raw materials, etc. High product purity and yield, improving purity and yield, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of o-isopropoxyphenol includes the following steps:
[0027] Add 225kg (2.0kmol) catechol, 450.0kg solvent 1,2-dimethoxyethane, 159.0kg (1.5kmol) sodium carbonate and 172.7kg (2.2kmol) 2-chloropropane into a 3000L autoclave After mixing uniformly, react at 170°C for 7 hours; after the reaction, the reaction product is cooled to 25°C and filtered to obtain the first filtrate and filter residue. The filter residue was washed with a solvent (1,2-dimethoxyethane) to obtain a second filtrate. The first filtrate and the second filtrate were mixed, firstly distilled under normal pressure, distilled out water and a small amount of solvent, and then rectified under reduced pressure. Collected the fraction product at 108℃~110℃ / 20mmHg to obtain o-isopropoxyphenol (279.2kg) . The fraction product was detected by high performance liquid chromatography, and the content of o-isopropoxyphenol was 99.3% (that is, the product purity was 99.3%). The yield based on cat...
Embodiment 2
[0029] A preparation method of o-isopropoxyphenol includes the following steps:
[0030] Add 225kg (2.0kmol) catechol, 337.5kg solvent 1-methoxy-2-ethoxyethane, 159.0kg (1.5kmol) sodium carbonate and 157kg (2.0kmol) 2-chloropropane to 3000L In the autoclave, the mixture was uniformly mixed and reacted at 170°C for 7 hours; after the reaction, the reaction product was cooled to 25°C and filtered to obtain the first filtrate and the filter residue. The filter residue was washed with a solvent (1-methoxy-2-ethoxyethane) to obtain a second filtrate. The first filtrate and the second filtrate were mixed, firstly distilled under normal pressure, distilled out water and a small amount of solvent, and then rectified under reduced pressure, collected the fraction product at 108℃~110℃ / 20mmHg to obtain o-isopropoxyphenol (278.1kg) . The fraction product was detected by high performance liquid chromatography, and the content of o-isopropoxyphenol was 99.0% (that is, the product purity was ...
Embodiment 3
[0032] A preparation method of o-isopropoxyphenol includes the following steps:
[0033] Add 225kg (2.0kmol) catechol, 382.5kg solvent 1-ethoxy-2-propoxyethane, 159.0kg (1.5kmol) sodium carbonate and 204.1kg (2.6kmol) 2-chloropropane to 3000L In the autoclave, mixed uniformly and reacted at 170°C for 7 hours; after the reaction, the reaction product was cooled to 25°C and filtered to obtain the first filtrate and filter residue. The filter residue was washed with a solvent (1-ethoxy-2-propoxyethane) to obtain a second filtrate. The first filtrate and the second filtrate were mixed, firstly distilled under normal pressure, water and a small amount of solvent were distilled out, and then rectified under reduced pressure, collecting the fraction product at 108℃~110℃ / 20mmHg to obtain o-isopropoxyphenol (280.0kg) . The fraction product was detected by high performance liquid chromatography, and the content of o-isopropoxyphenol was 99.4% (that is, the product purity was 99.4%). The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com