Azine-based CN-sensor molecule and synthesis and application thereof
A sensor and molecular technology, applied in the fields of chemical synthesis and anion detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the synthesis of sensor PX
[0038] Weigh (0.236g, 1.5mmol) quinoline-2-carbaldehyde and (0.204g, 1.5mmol) salicylaldehyde hydrazone respectively, dissolve them into a 50ml round bottom flask with 20ml DMF, add 0.3ml of glacial acetic acid as a catalyst, Heated to reflux at 85°C for 5 h, cooled to room temperature, added 5 ml of distilled water, a large amount of yellow precipitates precipitated, filtered, dried, and then recrystallized with DMF, after drying, the target product PX was obtained with a yield of 76%.
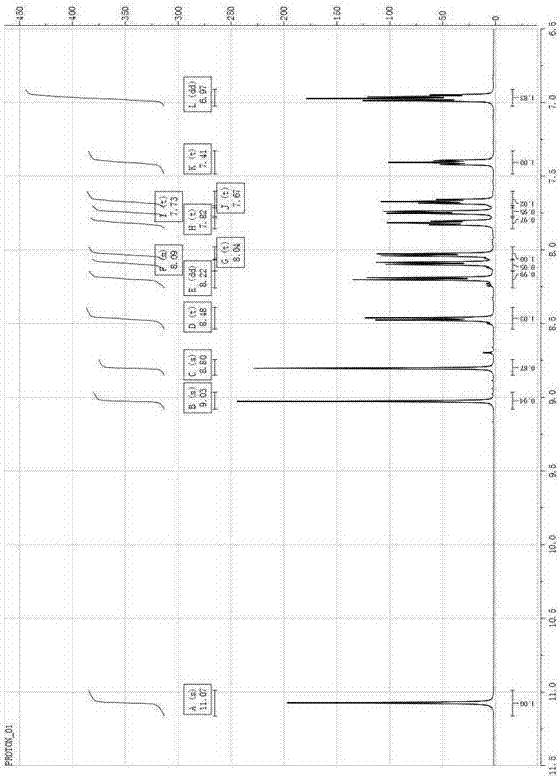
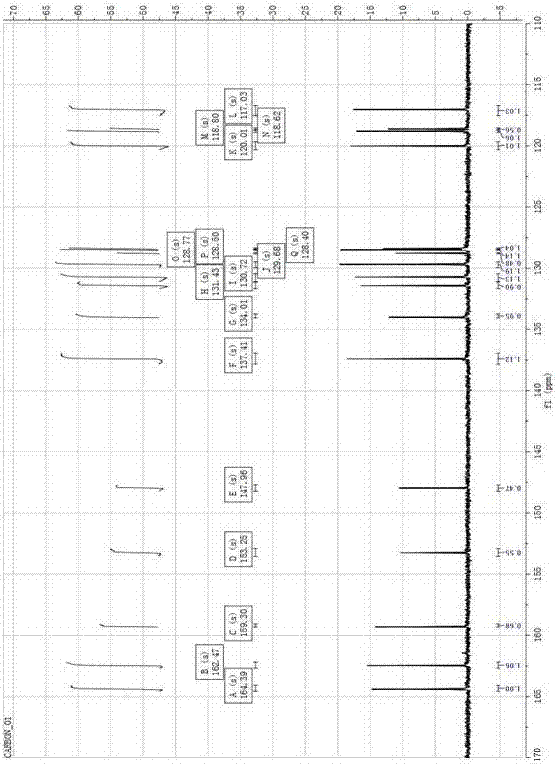
[0039] Color: yellow; Melting point: 164-166°C; 1 H-NMR (DMSO- d 6 , 400 MHz, ppm) δ: 11.07 (s,1H), 9.03 (s, 1H), 8.75 (s, 1H), 8.48(t, J=8.62 Hz, 1H), 8.22 (dd, J=8.33 Hz, 1H), 8.09 (m, 1H), 8.04(t, J=8.33Hz, 1H), 7.82(t, J=6.45 Hz 1H), 7.73(t, J=7.45 Hz, 1H), 7.67(t, J =6.97Hz, 1H), 7.41(t, J=7.24 Hz, 1H), 6.97(dd, J=7.99Hz, 2H). 13 C-NMR (DMSO- d 6 , 100 MHz, PPM) Δ: 164.39, 162.47, 157.30, 153.25,147.96, 137.41, 134.01, 131.43, 130....
Embodiment 2
[0040] Embodiment 2, CN - solution detection
[0041] Take 10 10 ml colorimetric tubes, add 0.5ml PX DMSO solution (2×10 -4 mol / L), and then add 0.5 ml anion (F − , Cl − , Br − , I − , AcO − , H 2 PO 4 − , HSO 4 − , ClO 4 − , SCN − and CN − ) in DMSO (10 -2 mol / L), if the color of the PX solution in the colorimetric tube changes from colorless to yellow, and under the ultraviolet light, the color of the PX solution in the colorimetric tube obviously changes from colorless to green, indicating that CN − , if the color of the PX solution in the colorimetric tube does not change significantly, and the fluorescence intensity of the PX solution does not change significantly under the ultraviolet light, it means that the addition of CN is not − .
Embodiment 3
[0042] Embodiment 3, CN - Production and application of test paper
[0043] Prepare PX with DMSO to 0.01 mol L −1 solution, take a piece of filter paper and cut it into two pieces with a length of about 3cm and a width of about 1cm, soak them in the DMSO solution of PX for 10 minutes, and then take them out to dry. The above filter paper was observed under ultraviolet light, no fluorescence was found on the filter paper.
[0044] Drop anion (F − , Cl − , Br − , I − , AcO − , H 2 PO 4 − , HSO 4 − , ClO 4 − , SCN − and CN − ) DMSO solution on the filter paper, if the color of the filter paper changes from colorless to yellow, and the color of the filter paper changes to green under the ultraviolet light, it means that the CN − , if the color of the filter paper does not change significantly under visible light and ultraviolet light, it means that other anions are added dropwise.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com