Process for synthesizing pyraclostrobin intermediate alpha-bromo-2-nitrotoluene
A technology of pyraclostrobin and nitrobenzyl bromide, which is applied in the synthesis process field of pyraclostrobin intermediate o-nitrobenzyl bromide, can solve the problems of unfavorable economy, low synthesis efficiency of o-nitrobenzyl bromide, Low yield and other problems, to achieve the effect of meeting the design of the synthesis process, optimizing the design of the synthesis process, and saving the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
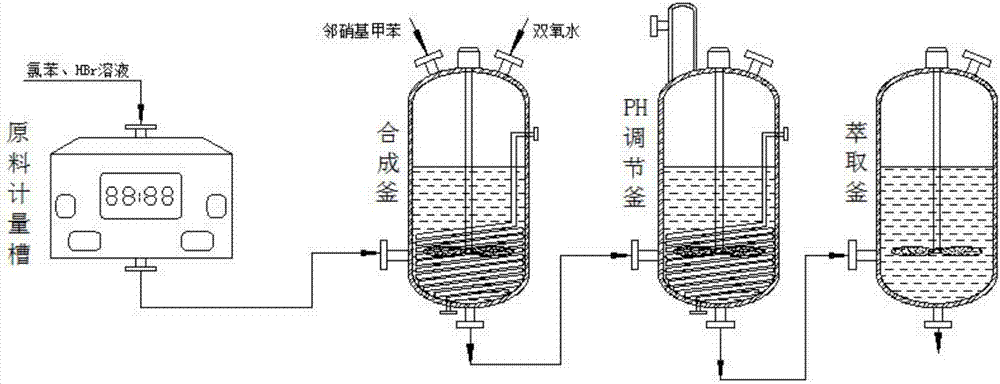
[0026] see figure 1 Shown, the technical scheme that the present invention adopts is: a kind of synthetic technique of pyraclostrobin intermediate o-nitrobenzyl bromide, and the concrete of described process method comprises the following steps:
[0027] (1). Raw material measurement: Use the raw material metering tank to measure quantitative chlorobenzene and HBr aqueous solution, and transfer the quantitative chlorobenzene and HBr aqueous solution to the interior of the o-nitrobenzyl bromide synthesis kettle for reaction;
[0028] (2). Oxidative synthesis: After setting the temperature rise parameters and stirring parameters in the o-nitrobenzyl bromide synthesis kettle, add o-nitrotoluene and hydrogen peroxide dropwise into the o-nitrobenzyl bromide synthesis kettle, turn on the stirrer to stir and keep warm for a period of time time;
[0029] (3).PH value adjustment: After the reaction inside the o-nitrobenzyl bromide synthesis kettle is completed, transfer the bottom mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com