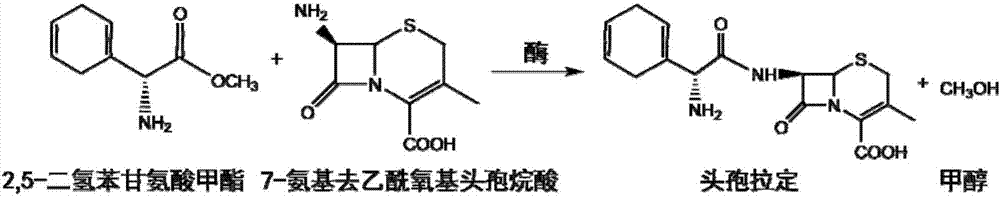
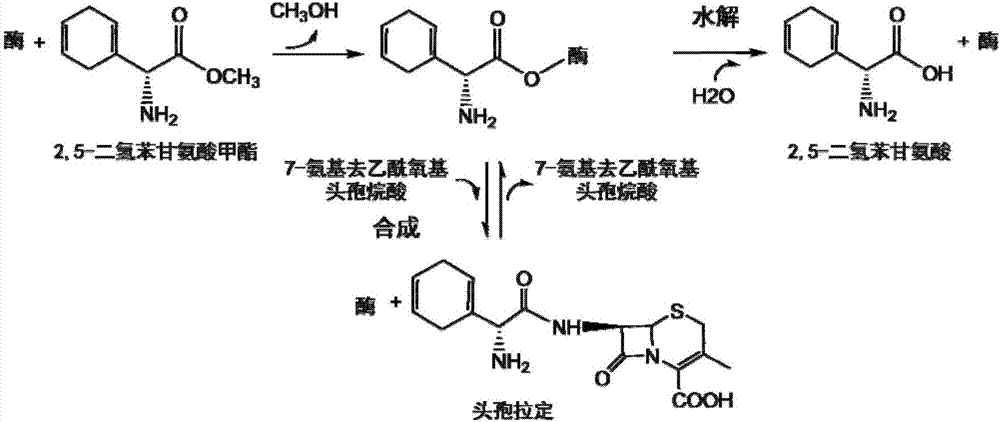
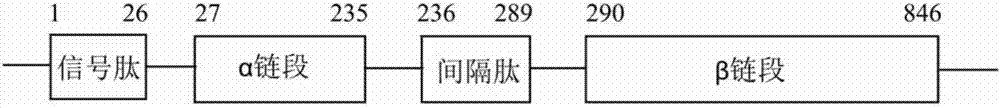
Cefradine synthase mutant and coding gene thereof
A cephradine and encoding technology, applied in the field of biochemistry, can solve the problems of not synthesizing cephradine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, preparation and purification of penicillin G acylase mutant
[0060] 1. Construction of gene encoding penicillin G acylase mutant and recombinant expression vector
[0061] The wild Escherichia coli penicillin G acylase gene was obtained according to literature. Sequence The pET28a-PGA_M1 gene (sequence 1) and pET28a-PGA_M2 gene (sequence 3) in the present invention were amplified by Overlapping PCR. Histidine tag His 6 -tag is added at the end of the carbon-terminus of the gene sequence to facilitate subsequent purification steps. After being double-digested and purified with NcoI and XhoI, it was ligated overnight with the expression vector pET28a(+) (containing the kanamycin resistance gene) that was double-cut with the same endonuclease, and then transformed into competent cells BL21( DE3), the recombinant expression plasmids pET28a-PGA_M1 and pET28a-PGA_M2 were obtained.
[0062] After sequencing, the sequences of pET28a-PGA_M1 and pET28a-PGA_M2 w...
Embodiment 2
[0076] Embodiment 2, the hydrolysis DHME catalytic activity assay of enzyme mutant
[0077] The DHME conversion product was analyzed by HPLC (LC-20AT, Shimadzu Corporation), so as to determine the catalytic activity and kinetic parameters of the hydrolysis of DHME by the two enzyme mutants PGA_M1 and PGA_M2 prepared in Example 1. The chromatographic column selected was an Inertsil C18 reverse-phase column (GL Sciences, 5 μm, 150×4.6 mm). During the reaction, the ratio of the enzyme mutant to the substrate was: 0.5mL of the purified target protein (sufficient, stored in 100mM potassium phosphate buffer, pH 7.0) and 0.5mL of DHME solution (pH 7.0, the concentration was gradient, The highest concentration is 1g / 100mL), react at 22°C for 8min. After the reaction was completed, 1 mL of methanol was added to terminate the reaction. The mobile phase ratio of HPLC is: 75% potassium phosphate buffer solution (30mM, pH 4.5): 25% methanol (v / v), flow rate 0.8mL / min, detection wavelengt...
Embodiment 3
[0082] Embodiment 3, the hydrolysis cephradine catalytic activity determination of enzyme mutant
[0083] The conversion product of cephradine was analyzed by HPLC (LC-20AT, Shimadzu Corporation), thereby determining the catalytic activity and kinetic parameters of the hydrolysis of cephradine by the two enzyme mutants PGA_M1 and PGA_M2 prepared in Example 1. The selected chromatographic column is an Inertsil C18 reverse-phase column (GL Sciences, 5 μm, 150×4.6 mm). During the reaction process, the ratio of enzyme mutant to substrate is: 0.5mL purified target protein (sufficient, stored in 100mM potassium phosphate buffer, pH 7.0) and 0.5mL cephradine solution (pH 7.0, the concentration is a gradient change, The highest concentration is 2g / 100mL), react at 22°C for 8min. After the reaction was completed, 1 mL of methanol was added to terminate the reaction. The mobile phase ratio of HPLC is: 75% potassium phosphate buffer solution (30mM, pH 4.5): 25% methanol (v / v), flow rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com