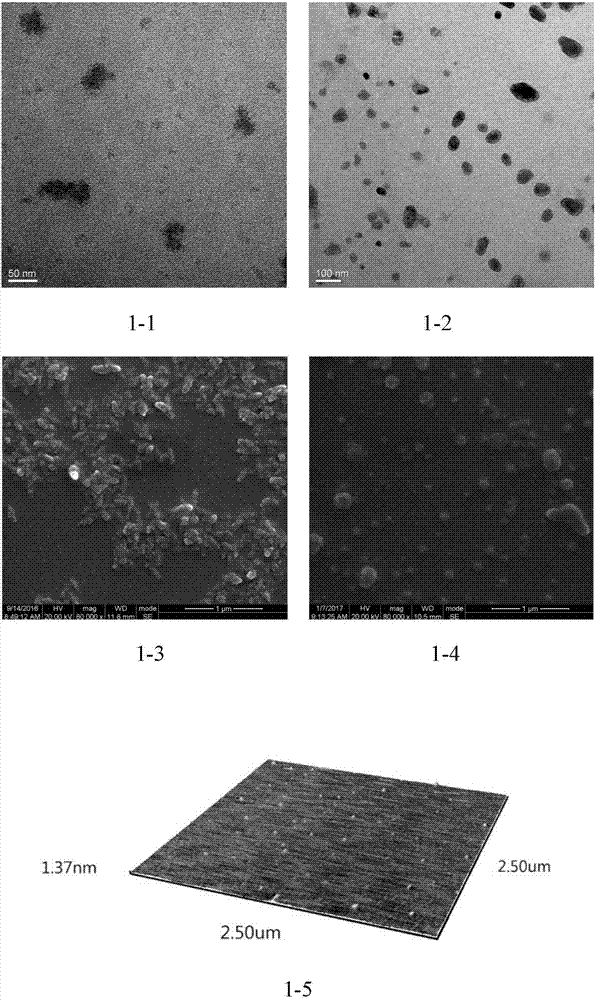
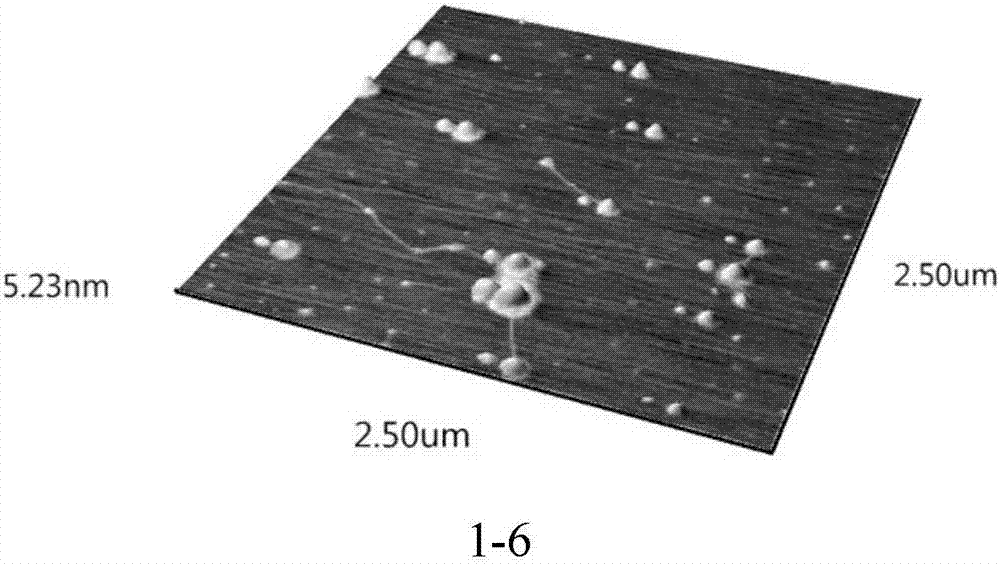
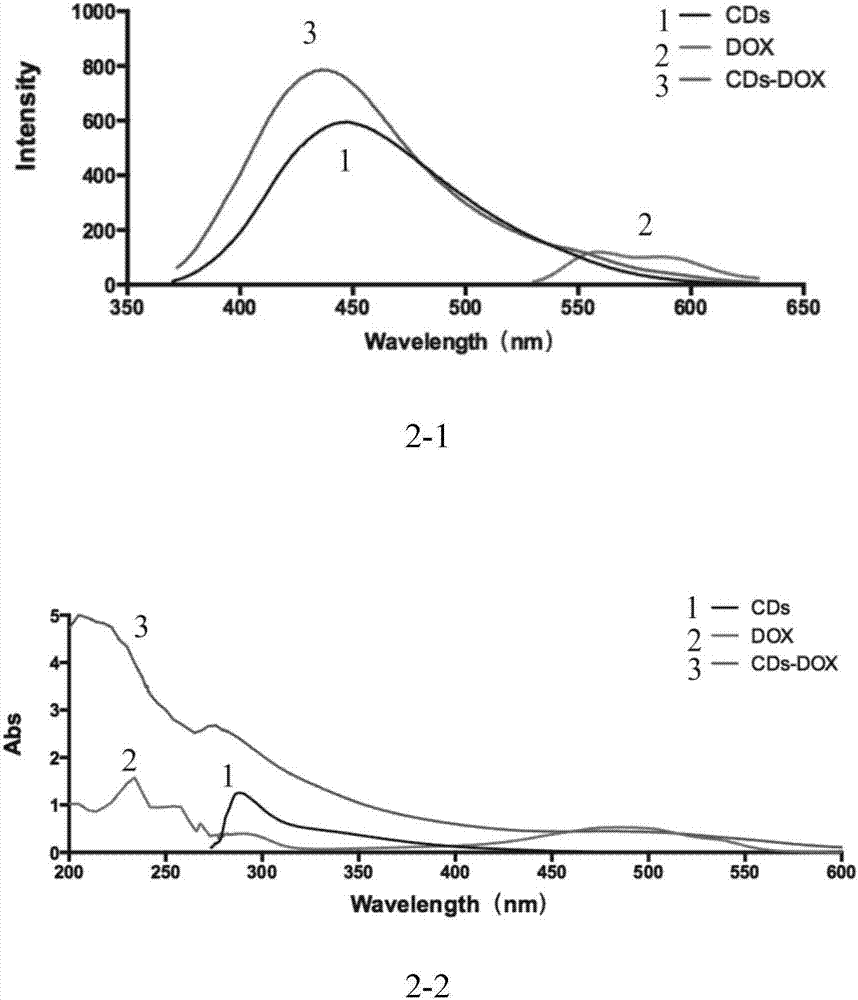
Bio-safety carbon quantum dot-loaded adriamycin complex as well as preparation method and application thereof
A biosafety, carbon quantum dot technology, applied in the field of medicine, can solve the problems of complex preparation process and potential safety hazards in the drug-loading system, and achieve the effects of simple synthesis process, low preparation cost and implementation conditions, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A biosafety carbon quantum dot-loaded doxorubicin complex, the preparation method of which comprises the following steps:
[0033] (1) Mix commercially available milk and deionized water at a volume ratio of 5:4, then heat at 180°C for 2 hours;
[0034] (2) Cool the solution obtained in step (1) to room temperature naturally, then centrifuge at 12000r / min for 30min, and take the supernatant;
[0035] (3) filtering the supernatant obtained in step (2) with a filter membrane of 0.22 μm to obtain a carbon quantum dot solution;
[0036] (4) vacuum lyophilize the carbon quantum dot solution obtained in step (3), then add deionized water to make a 12mg / mL aqueous solution;
[0037] (5) Dissolving doxorubicin hydrochloride powder in deionized water to make a 400 μg / mL aqueous solution;
[0038] (6) mixing the aqueous solution of carbon quantum dots obtained in step (4), the aqueous solution of doxorubicin obtained in step (5) and the PBS solution in a volume ratio of 1:1:2 t...
Embodiment 2
[0044] A biosafety carbon quantum dot-loaded doxorubicin complex, the preparation method of which comprises the following steps:
[0045] (1) Mix commercially available milk and deionized water at a volume ratio of 1:1, then heat at 180°C for 2 hours;
[0046] (2) Naturally cool the solution obtained in step (1) to room temperature, then centrifuge at 12000r / min for 45min, and take the supernatant;
[0047] (3) filtering the supernatant obtained in step (2) with a filter membrane of 0.22 μm to obtain a carbon quantum dot solution;
[0048] (4) vacuum lyophilize the carbon quantum dot solution obtained in step (3), then add deionized water to make a 12mg / mL aqueous solution;
[0049] (5) Dissolving doxorubicin hydrochloride powder in deionized water to make a 400 μg / mL aqueous solution;
[0050] (6) The carbon quantum dot aqueous solution obtained in step (4), the adriamycin aqueous solution obtained in step (5) and the PBS solution are mixed in a volume ratio of 1:2:5 to mak...
Embodiment 3
[0054] A biosafety carbon quantum dot-loaded doxorubicin complex, the preparation method of which comprises the following steps:
[0055] (1) Mix commercially available milk and deionized water at a volume ratio of 5:4, then heat at 180°C for 2 hours;
[0056] (2) Cool the solution obtained in step (1) to room temperature naturally, then centrifuge at 12000r / min for 30min, and take the supernatant;
[0057] (3) filtering the supernatant obtained in step (2) with a filter membrane of 0.22 μm to obtain a carbon quantum dot solution;
[0058] (4) vacuum lyophilize the carbon quantum dot solution obtained in step (3), then add deionized water to make a 12mg / mL aqueous solution;
[0059] (5) Dissolving doxorubicin hydrochloride powder in deionized water to make a 400 μg / mL aqueous solution;
[0060] (6) mixing the aqueous solution of carbon quantum dots obtained in step (4), the aqueous solution of doxorubicin obtained in step (5) and the PBS solution in a volume ratio of 3:2:3 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com