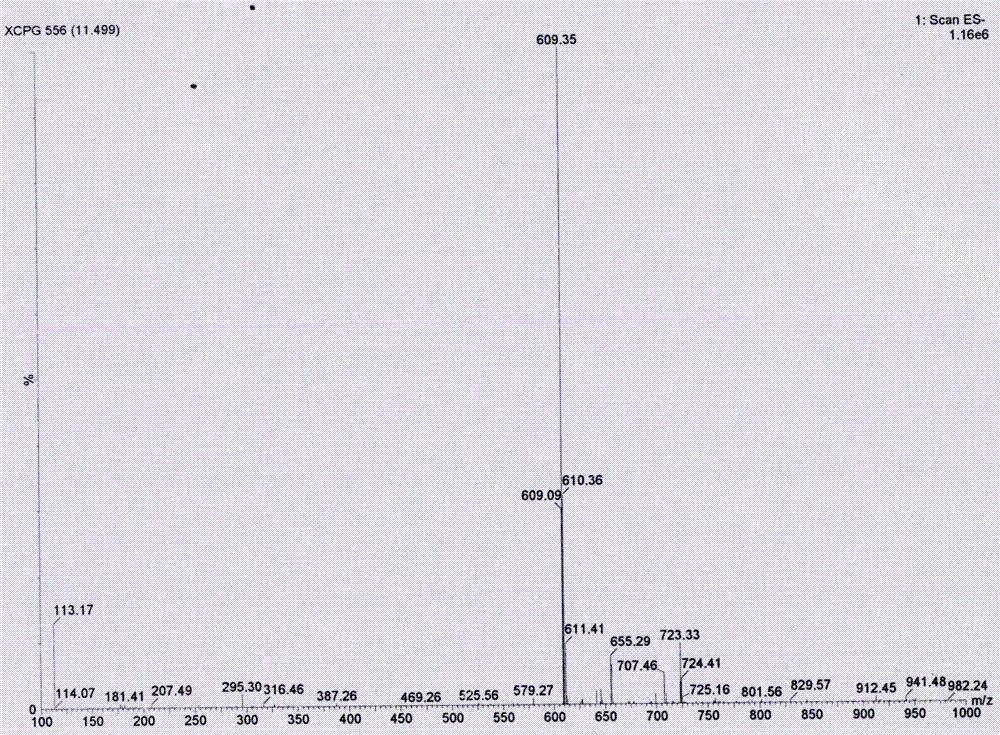
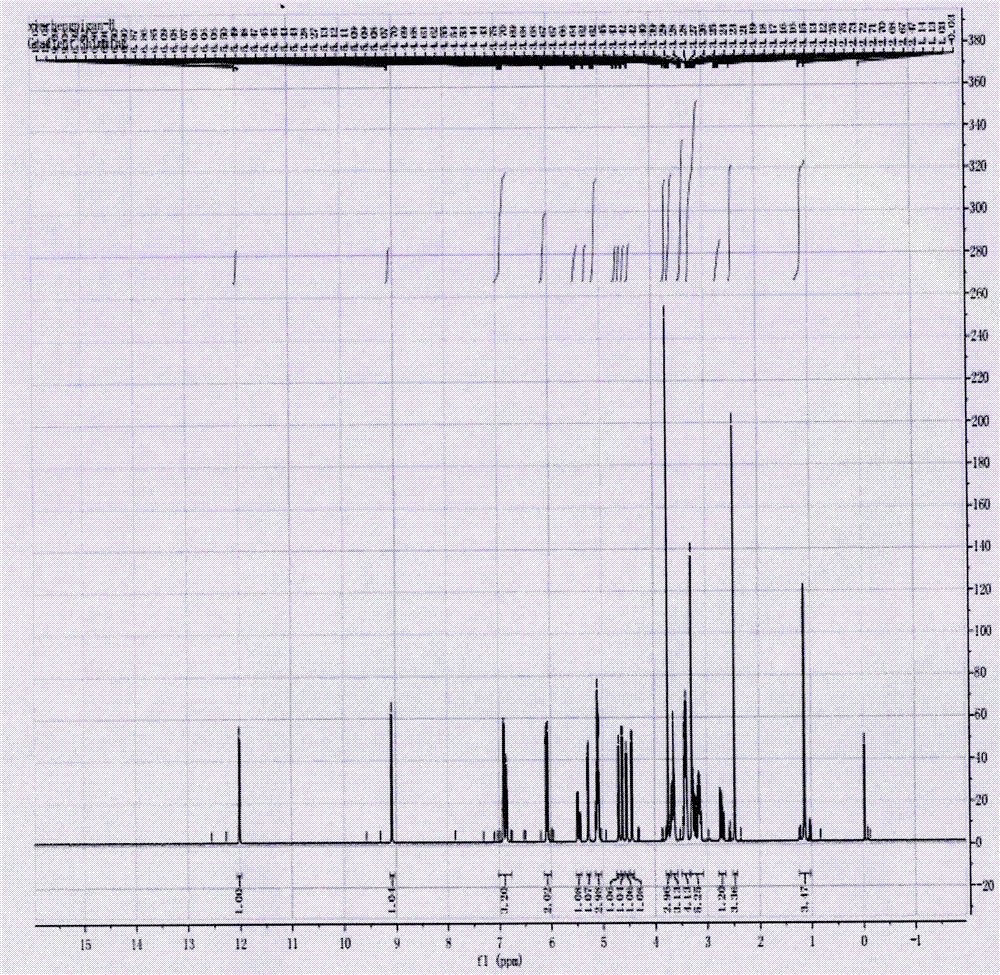
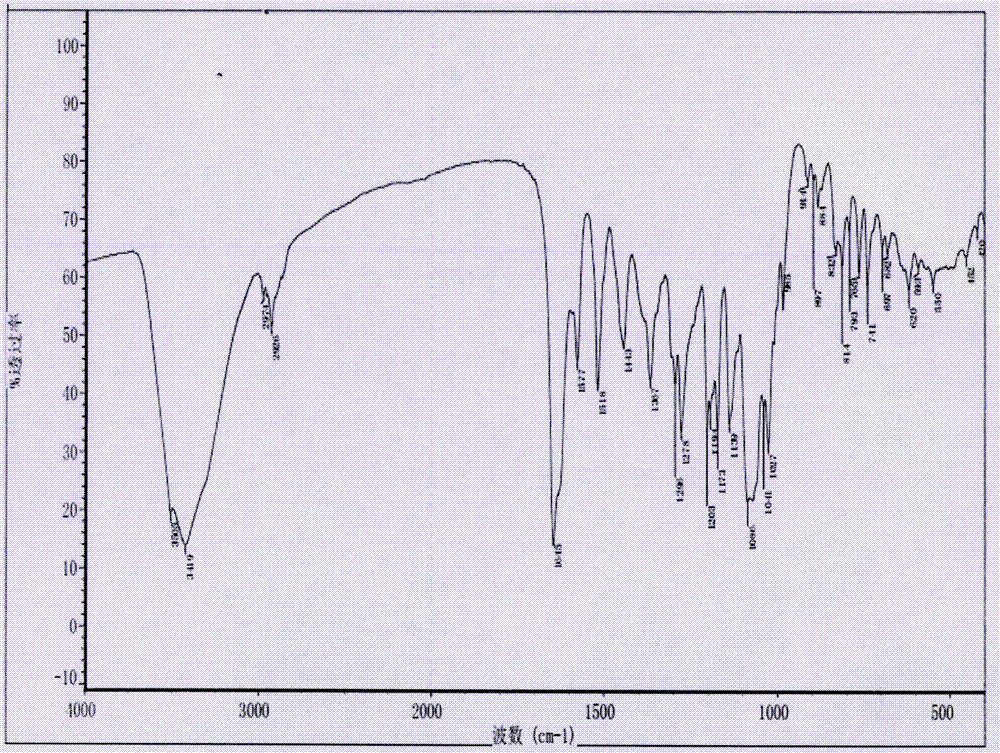
Synthesis process of neohesperidin
A new hesperidin and synthetic process technology, applied in the direction of organic chemistry, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult separation of catalysts and solvents, inability to purify intermediate products, and low product quality, so as to achieve the goal of protecting Vascular system, short reaction time, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A kind of synthetic technique of neohesperidin, is characterized in that, comprises the following steps:
[0056] 1) Preparation of root bark acetophenone-4′-β-neohesperidoside
[0057] After adding 8g of naringin into the reactor, add 96mL of potassium hydroxide solution with a mass fraction of 17.5% in the reactor; feed nitrogen into the reactor to drive out the air in the reactor, and then heat to 100 ℃, obtained mixture A after reflux reaction for 2h;
[0058] 2) Purification of root bark acetophenone-4′-β-neohesperidoside
[0059] 2.1) Put the mixture A in an ice bath, adjust the pH of the mixture A to 6±0.5 with concentrated hydrochloric acid, precipitate a light yellow crystal, heat above 70°C until the crystals are completely dissolved, and obtain the mixture B;
[0060] The concentration range of the concentrated hydrochloric acid is 36%.
[0061] 2.2) Let the mixture B stand at room temperature for 2 hours, crystallize after cooling, filter under reduced pr...
Embodiment 2
[0077] A kind of synthetic technique of neohesperidin, is characterized in that, comprises the following steps:
[0078] 1) Preparation of root bark acetophenone-4′-β-neohesperidoside
[0079] After adding 8g of naringin into the reactor, add 64mL of potassium hydroxide solution with a mass fraction of 20% in the reactor; feed nitrogen into the reactor to drive out the air in the reactor, and then heat to 100 ℃, obtained mixture A after reflux reaction for 2h;
[0080] 2) Purification of root bark acetophenone-4′-β-neohesperidoside
[0081] 2.1) Put the mixture A in an ice bath, adjust the pH of the mixture A to 6±0.5 with concentrated hydrochloric acid, precipitate a light yellow crystal, heat above 70°C until the crystals are completely dissolved, and obtain the mixture B;
[0082] The concentration range of the concentrated hydrochloric acid is 37%.
[0083] 2.2) Let the mixture B stand at room temperature for 3 hours, crystallize after cooling, filter under reduced pres...
Embodiment 3
[0099] A kind of synthetic technique of neohesperidin, is characterized in that, comprises the following steps:
[0100] 1) Preparation of root bark acetophenone-4′-β-neohesperidoside
[0101] After adding 8g of naringin into the reactor, add 96mL of potassium hydroxide solution with a mass fraction of 22.5% in the reactor; feed nitrogen into the reactor to drive out the air in the reactor, and then heat to 100 °C, the mixture A was obtained after reflux reaction for 1.5h;
[0102] 2) Purification of root bark acetophenone-4′-β-neohesperidoside
[0103] 2.1) Put the mixture A in an ice bath, adjust the pH of the mixture A to 6±0.5 with concentrated hydrochloric acid, precipitate a light yellow crystal, heat above 70°C until the crystals are completely dissolved, and obtain the mixture B;
[0104] The concentration range of the concentrated hydrochloric acid is 38%.
[0105] 2.2) Let the mixture B stand at room temperature for 4 hours, crystallize after cooling, filter under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com