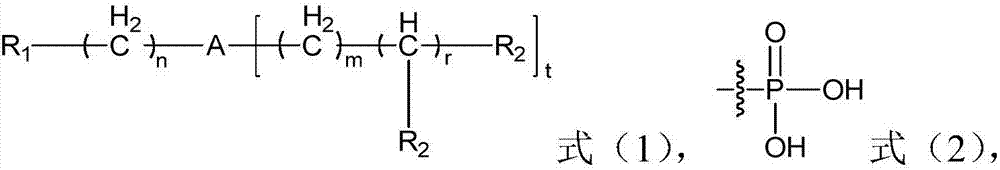
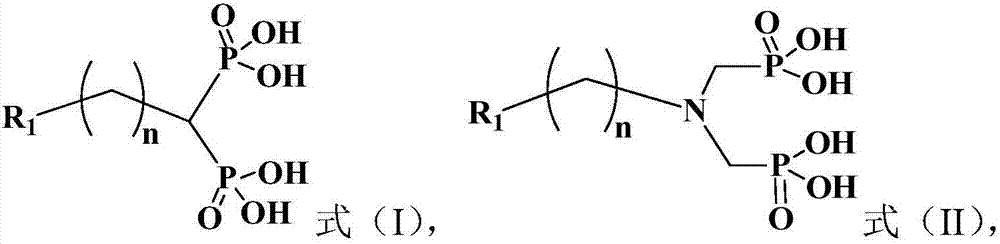Application of organic phosphonate compound, rubidium-doped perovskite solar battery thin film and preparation method therefor
A solar cell and organic phosphonic acid technology, which can be used in circuits, photovoltaic power generation, electrical components, etc., can solve the problems that limit the development of perovskite batteries, unstable performance of methylamine lead iodine perovskite, and easy hydrolysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
[0074] The method for preparing the compound shown in formula (II), comprising: in the presence of concentrated hydrochloric acid, the compound shown in formula (II-VI) is carried out reflux reaction with phosphorous acid and formaldehyde donor; Preferably said formaldehyde donor is able to provide Substances with formaldehyde structure,
[0075]
[0076] R in formula (II) and formula (II-VI) 1 The definitions of and n are the same as the aforementioned definitions of the present invention.
specific Embodiment approach 3
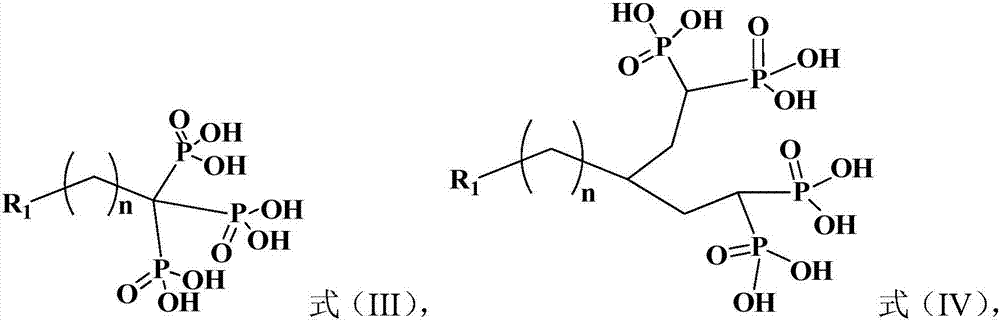
[0078] The method for preparing the compound shown in formula (III), comprising: reacting the compound shown in formula (III-VII) with sodium hexamethyldisilazide and diethylphosphorous oxychloride, and then performing hydrolysis;
[0079]
[0080] R in formula (III) and formula (III-VII) 1 The definition of and n is the same as the aforementioned definition of the present invention; R in the formula (III-VII) 3 for C 1-8 The alkyl group, more preferably methyl, ethyl, isopropyl, n-butyl and tert-butyl, particularly preferably isopropyl.
specific Embodiment approach 4
[0082] The method for preparing the compound shown in formula (IV), comprising: reacting the compound shown in formula (IV-VIII) with tetraester of methine diphosphonate, and then performing hydrolysis;
[0083]
[0084] R in formula (IV) and formula (IV-VIII) 1 The definition of and n is the same as the aforementioned definition of the present invention; X in the formula (IV-VIII) 2 is a halogen element, preferably chlorine, bromine or iodine, more preferably bromine.
[0085] Those skilled in the art can easily obtain the specific preparation of the organic phosphonic acid compound shown in formula (1) claimed in the present invention according to the method provided in the above specific embodiment and the exemplary preparation method provided in the preparation example of the present invention Methods, the present invention does not state the preparation methods for each specific compound one by one, those skilled in the art should not understand it as a limitation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com