Sample for verifying total number counting capability of aerobic bacteria in drug and preparation method thereof
A technology of proficiency testing and aerobic bacteria, applied in the direction of biochemical equipment and methods, microbiological determination/inspection, etc., can solve problems such as fatal risk and patient infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Samples for proficiency verification of the total number of aerobic bacteria in medicines, the target bacteria include: Staphylococcus aureus, Bacillus cereus, Escherichia coli (E.coil), Klebsiella pneumoniae ( Klebsiella pneumoniae) and Citrobacter freundii.
[0030] The sample is based on trehalose, skimmed milk powder and sterile water, wherein the volume fraction of trehalose is 12%, and the volume fraction of skimmed milk powder is 0.5%.
[0031] The concentration of the target flora in the sample is 10 2 ~10 3 CFU / mL.
Embodiment 2
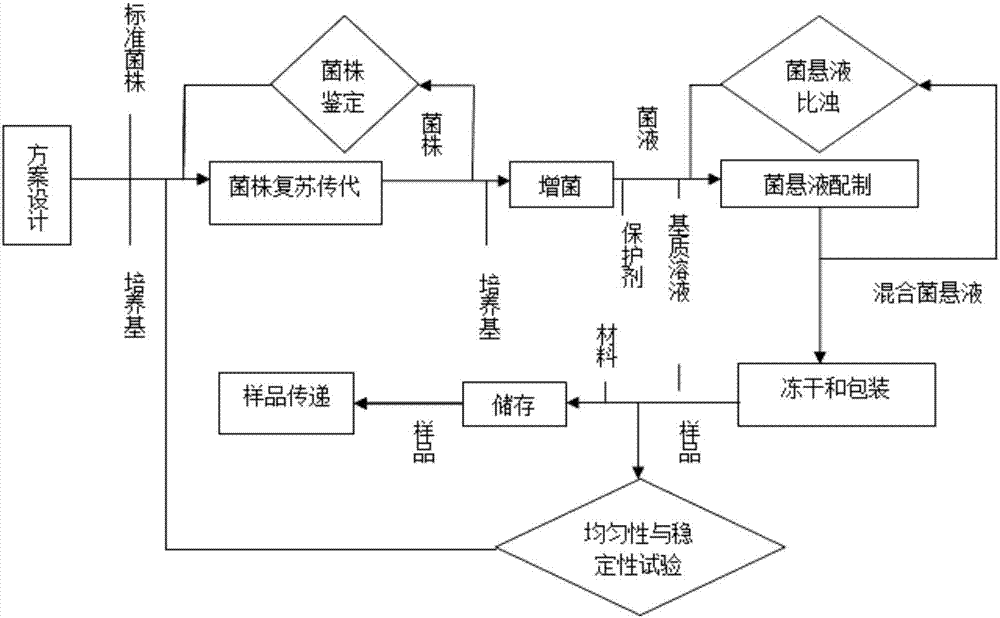
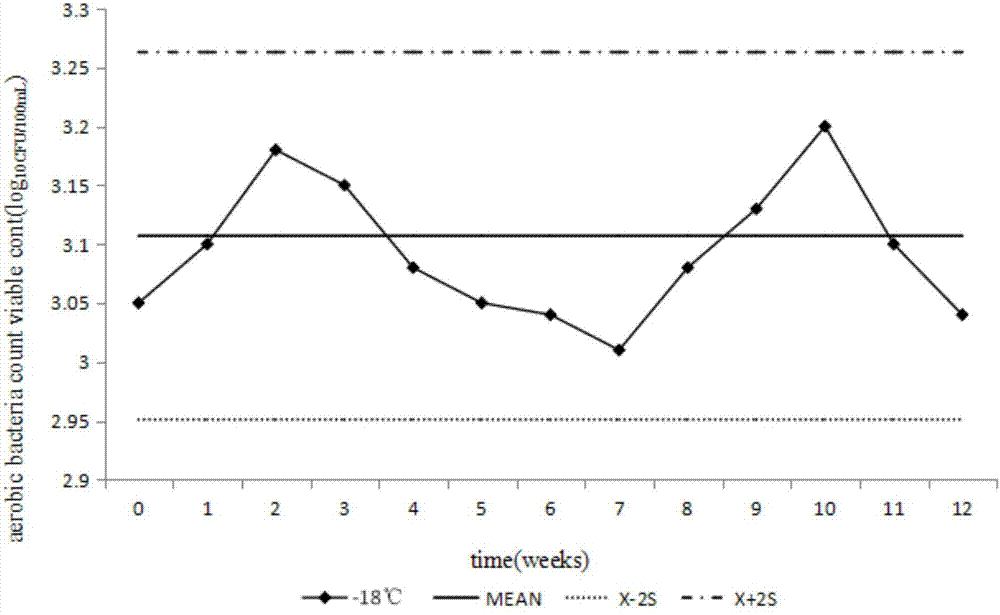
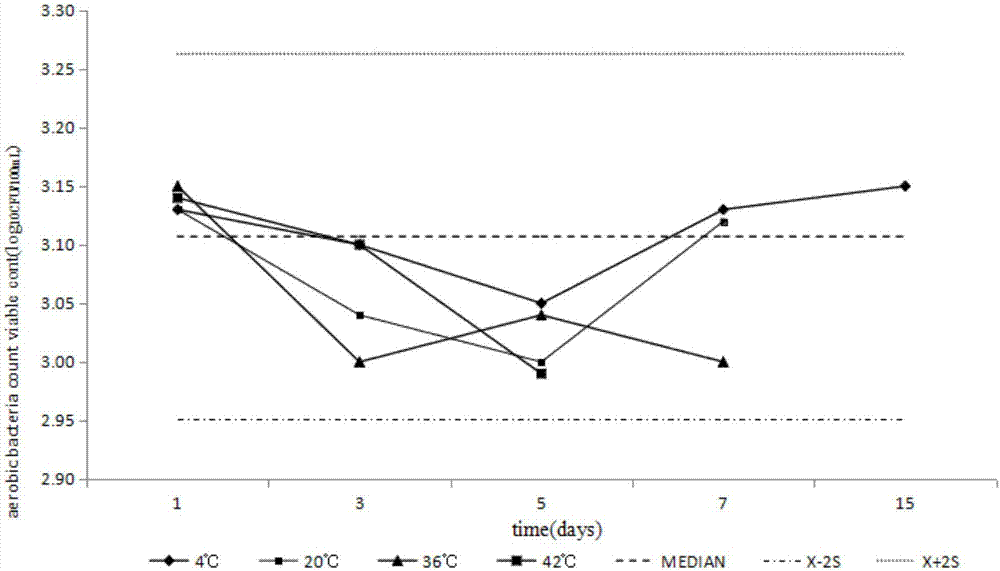
[0033] Such as figure 1 As shown, a method for preparing a test sample for counting the total number of aerobic bacteria in medicines in Example 1, including the selection of sample addition bacterial strains, preparation of freeze-drying protective agent, sample freeze-drying, sample uniformity and stability test IV steps, the specific steps are as follows:
[0034] 1. Selection of sample addition strains
[0035] According to target flora: Staphylococcus aureus, Bacillus cereus, Escherichia coli (E.coil), Klebsiella pneumoniae (Klebsiella pneumoniae) and Citrobacter freundii ), all standard strains were purchased from institutions designated by the government, and a strain certificate was attached to ensure the traceability of the strains.
[0036] 2. Preparation of lyoprotectant
[0037] The sample was prepared with trehalose, skimmed milk powder and sterilized water as the matrix (volume fraction: trehalose 12%, skimmed milk powder 0.5%) to prepare a lyoprotectant.
[...
Embodiment 3
[0070] Each step of the preparation method of the proficiency verification sample for counting the total number of aerobic bacteria in the medicine described in this embodiment is all the same as in Example 2, and the different technical parameters are: in the preparation of the bacterial suspension, each in the target bacterial suspension The concentration of the target bacteria was 5×10 3 CFU / mL preparation; freeze-drying process 45h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com