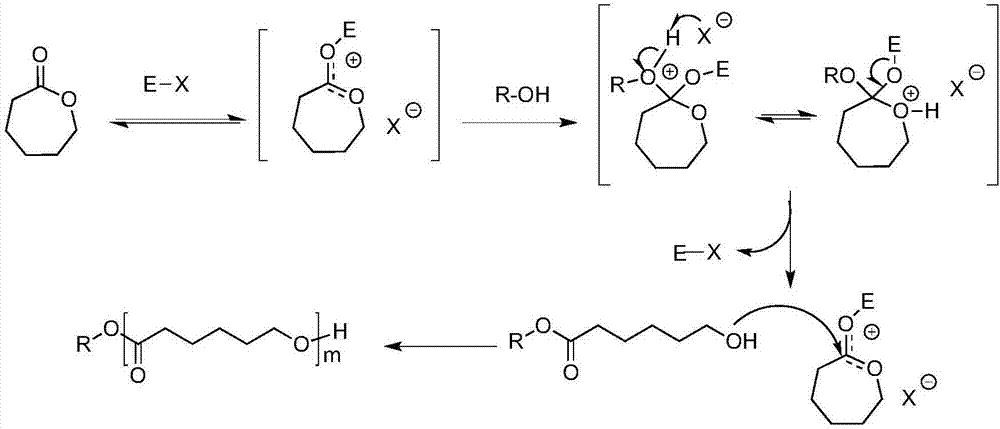
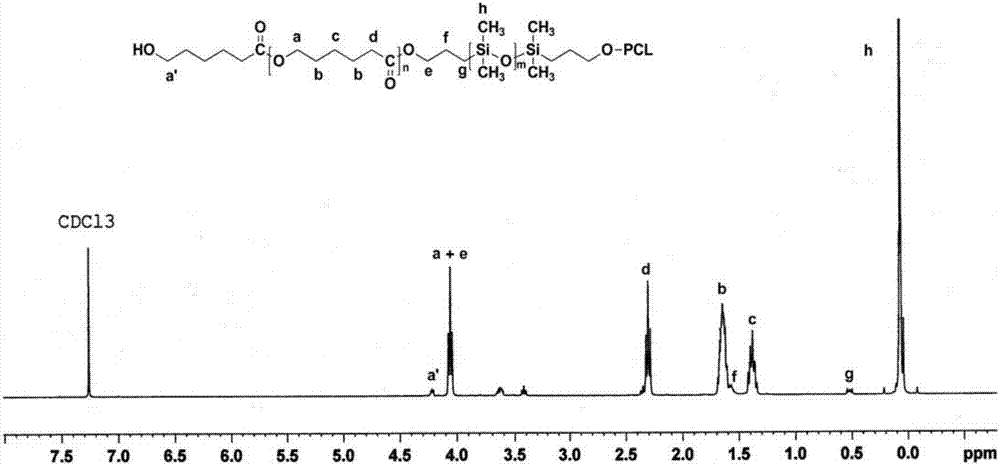
Method for preparing PCL-PDMS-PCL stabilizing agent by hydrogen chloride/ether solution catalysis
A PCL-PDMS-PCL, catalytic preparation technology, applied in the production of bulk chemicals, etc., can solve the problems of toxic side effects, long reaction time, etc., achieve mild reaction conditions, shorten reaction time, and avoid toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 5 g (0.0015 mol) of HTPDMS (0.0015 mol) that has been weighed, 30 mL of dichloromethane that has been dried and dehydrated, and 5 g (0.044 mol) of ε-CL into a 50 mL three-necked round-bottomed flask, and then add 4.2 mol / L of HCl·Et 2 O solution 0.71mL (0.003mol), stirred at 25°C for 4 hours, and protected by argon; after the reaction was completed, added to 300mL of cold methanol, filtered after the product was precipitated, and dried in vacuo to obtain the product PCL-PDMS-PCL8.6g, The molecular weight is 6384 g / mol, the yield is 86%.
Embodiment 2
[0022] Add 5 g (0.0015 mol) of HTPDMS (0.0015 mol) that has been weighed, 30 mL of dichloromethane that has been dried and dehydrated, and 5 g (0.044 mol) of ε-CL into a 50 mL three-necked round-bottomed flask, and then add 4.2 mol / L of HCl·Et 2 O solution 1.42mL (0.006mol), stirred at 25°C for 4 hours, and protected by argon; after the reaction was completed, added to 300mL of cold methanol, filtered after the product was precipitated, and dried in vacuo to obtain the product PCL-PDMS-PCL8.5g, The molecular weight is 6016 g / mol, the yield is 85%.
Embodiment 3
[0024] Add 5 g (0.0015 mol) of HTPDMS (0.0015 mol) that has been weighed, 30 mL of dichloromethane that has been dried and dehydrated, and 5 g (0.044 mol) of ε-CL into a 50 mL three-necked round-bottomed flask, and then add 4.2 mol / L of HCl·Et 2 O solution 0.71mL (0.003mol), stirred at 35°C for 4 hours, and protected by argon; after the reaction, added to 300mL of cold methanol, filtered after the product was precipitated, and dried in vacuo to obtain the product PCL-PDMS-PCL7.8g, The molecular weight is 6023 g / mol, the yield is 78%.
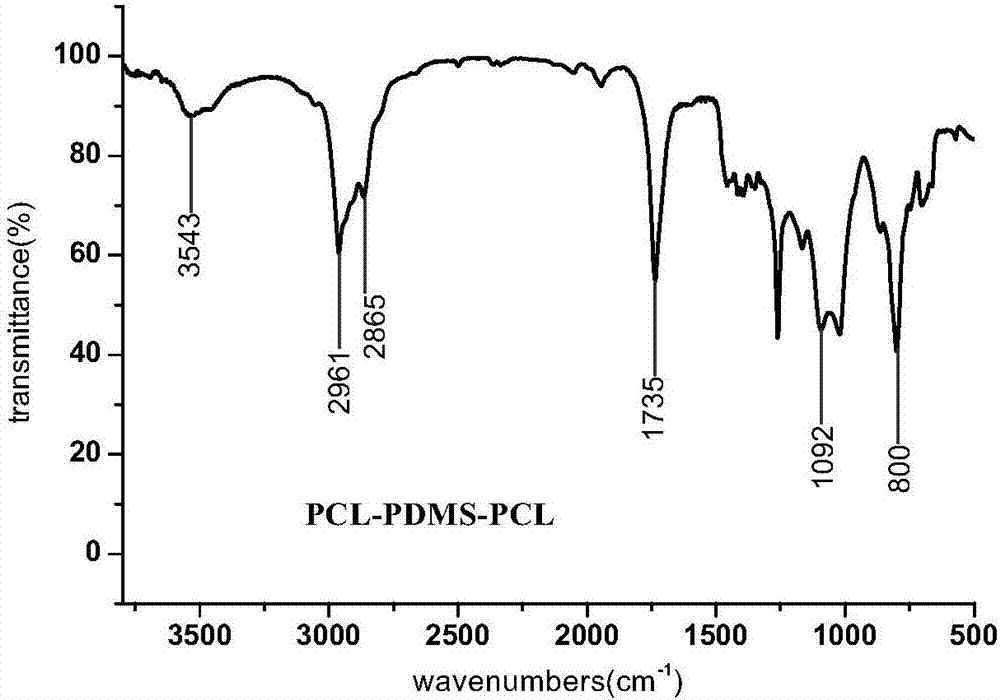
[0025] figure 2 It is the infrared spectrogram of the triblock stabilizer PCL-PDMS-PCL obtained in Example 1. at 3543cm -1 It is the stretching vibration peak of the terminal hydroxyl group (-OH) of the product, at 2961cm -1 and 2865cm -1 It is the C-H stretching vibration peak of the methylene group of PCL in PCL-PDMS-PCL, at 1735cm -1 It is the stretching vibration peak of the carbonyl group (C=O) in PCL, at 800cm -1 is the stretching v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com