Corrosion-resistant and wettability-perfect lead-free solder and preparation method thereof
A lead-free solder, wettability technology, applied in the direction of welding equipment, welding/cutting medium/material, welding medium, etc., can solve the problems of corrosion resistance not meeting the use requirements, solder joint corrosion damage, rough surface, etc. Achieve the effects of excellent physical and mechanical properties, refine grain structure and improve corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of Sn-Cu-Al matrix alloy
[0024] (1) Weigh respectively 0.75wt% of Cu powder and 0.075wt% of Al powder according to the weight percentage, and the balance is Sn powder, put them into a ball mill jar, vacuumize the ball mill jar and fill it with argon, and use the following parameters for ball milling and mixing : Speed 90r / min, ball-to-material ratio 10:1, ball milling time 25min. Press the mixed powder into tablets with a pressure of 10MPa and hold the pressure for 5 minutes, then put the metal tablets into a non-consumable vacuum electric arc furnace with a pre-vacuum degree of 4×10 -3 Pa, then filled with high-purity argon, and started melting, the melting voltage was 220V, the melting current was controlled at 1A, and the melting time was kept at 50s to obtain a Sn-Cu-Al matrix alloy. This alloy is designated as Sample 1.
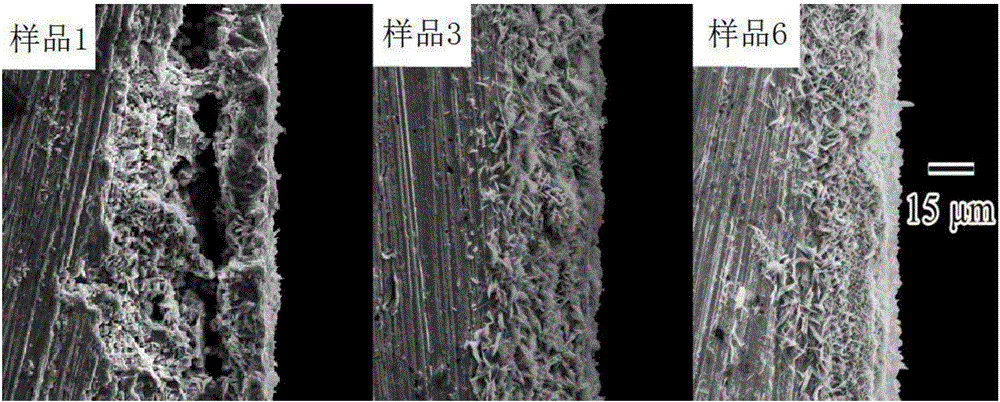
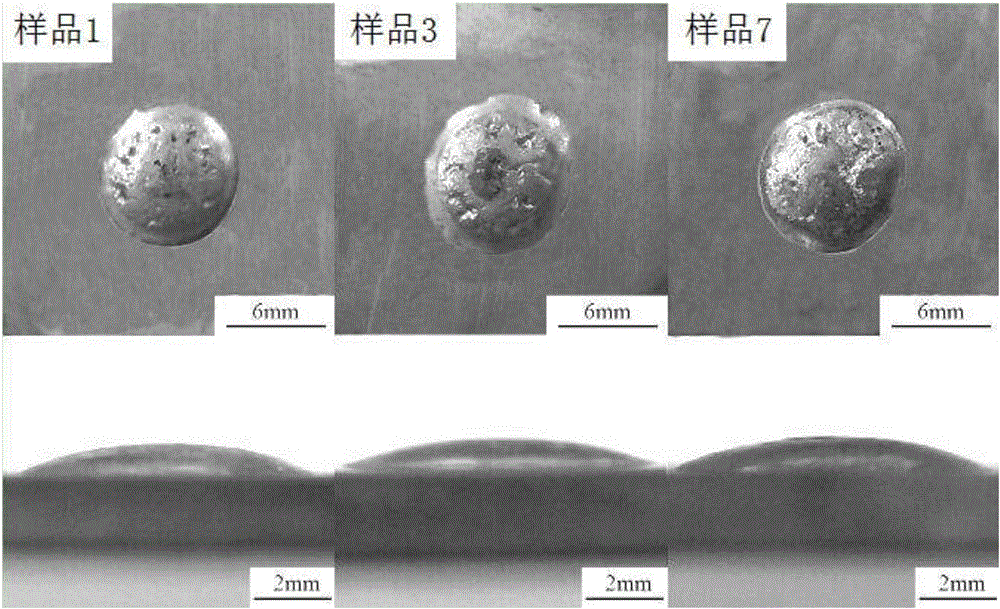
[0025] 2. Performance testing
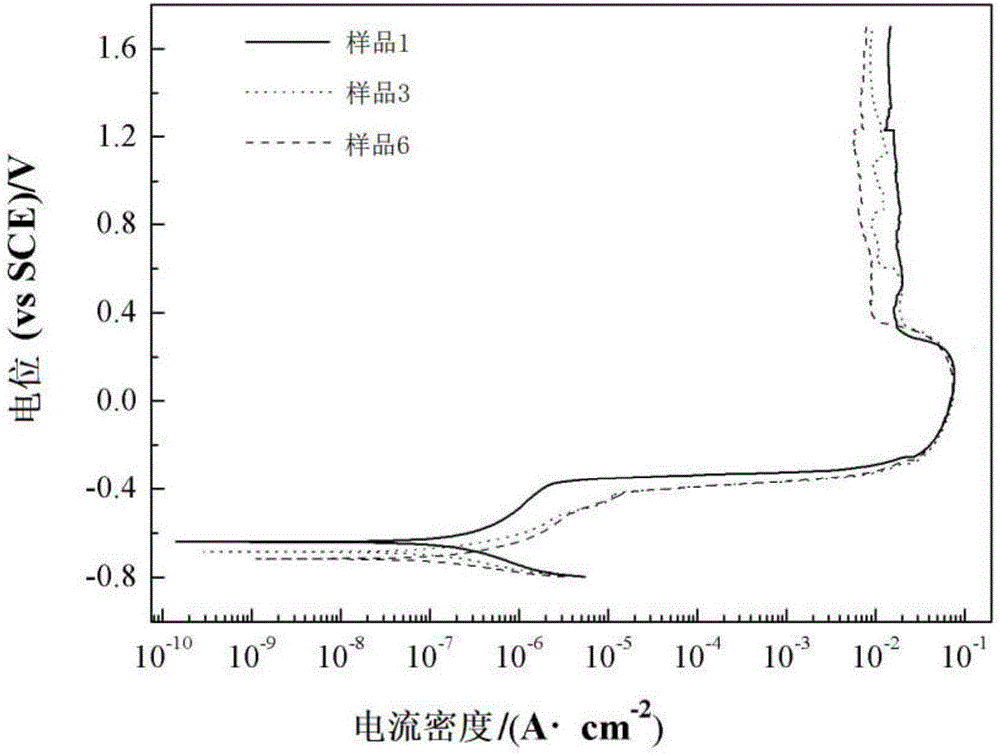
[0026] The polarization curve of sample 1 was tested with an electrochemical workstation, t...
Embodiment 2
[0029] 1. Preparation of lead-free solder with good corrosion resistance and wettability
[0030] (1) Weigh respectively 0.5wt% of Cu powder and 0.08wt% of Al powder according to the weight percentage, and the balance is Sn powder, put them into a ball mill jar, vacuumize the ball mill jar and fill it with argon, and use the following parameters for ball milling and mixing : Speed 150r / min, ball-to-material ratio 10:1, ball milling time 20min. Press the mixed powder into tablets with a pressure of 12 MPa and a holding time of 10 minutes, then put the metal tablets into a non-consumable vacuum electric arc furnace with a pre-vacuum degree of 6×10 -3 Pa, and then fill it with high-purity argon, start melting, the melting voltage is 220V, the melting current is controlled at 2A, the melting time is kept at 30s, and the Sn-Cu-Al matrix alloy is obtained.
[0031] (2) According to the weight percentage of 22.02wt%, 77.98wt%, respectively weigh blocky Sn and blocky rare earth Ce,...
Embodiment 3
[0037] 1. Preparation of lead-free solder with good corrosion resistance and wettability
[0038] (1) Weigh respectively 0.75wt% of Cu powder and 0.075wt% of Al powder according to the weight percentage, and the balance is Sn powder, put them into a ball mill jar, vacuumize the ball mill jar and fill it with argon, and use the following parameters for ball milling and mixing : Speed 90r / min, ball-to-material ratio 10:1, ball milling time 25min. Press the mixed powder into tablets with a pressure of 10MPa and hold the pressure for 5 minutes, then put the metal tablets into a non-consumable vacuum electric arc furnace with a pre-vacuum degree of 4×10 -3 Pa, and then fill it with high-purity argon, start melting, the melting voltage is 220V, the melting current is controlled at 1A, and the melting time is kept at 60s to obtain the Sn-Cu-Al matrix alloy.
[0039] (2) According to the weight percentage of 22.02wt%, 77.98wt%, respectively weigh blocky Sn and blocky rare earth Ce,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Corrosion current density | aaaaa | aaaaa |
| Passivation current density | aaaaa | aaaaa |
| Corrosion current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com