Solution preparation for aerosol inhalation for treating child cough and preparation method thereof
A technology for atomized inhalation and infantile cough and asthma, applied in the field of pharmacy, can solve the problems of poor compliance, low bioavailability, and slow onset of oral liquid, etc. The effect of the drug route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
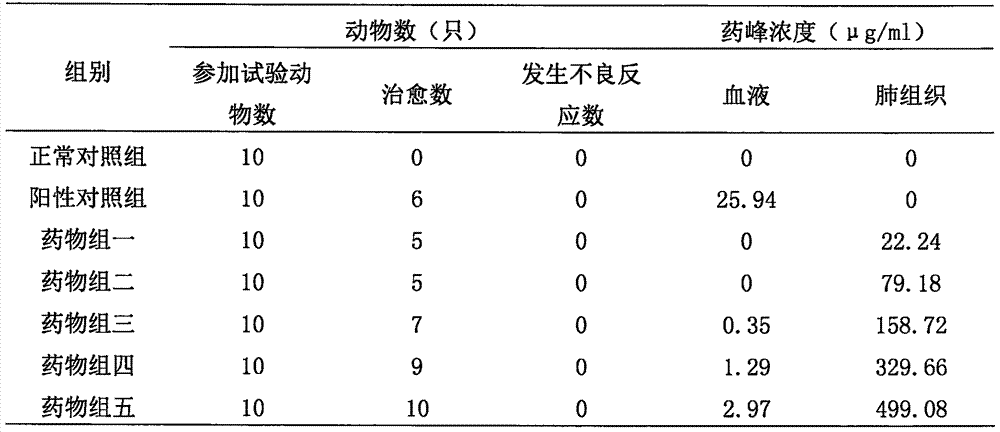
Image
Examples
Embodiment 1
[0030] (1) Preparation of active ingredients:
[0031] 50 parts of ephedra, 100 parts of bitter almond, 400 parts of gypsum, 50 parts of licorice, 167 parts of honeysuckle, 167 parts of forsythia, 167 parts of Anemarrhena, 167 parts of scutellaria, 167 parts of Radix Radix, 167 parts of Ophiopogon japonicus, 167 parts of Houttuynia cordata
[0032] Weigh the eleven herbs according to the above weight parts, add water to the gypsum and decoct for 0.5 hours, add the remaining ten herbs such as ephedra, add water and decoct twice, each time for 1 hour, combine the decoctions, filter, and concentrate the filtrate to a relative density of 80°C 1.10-1.15, let it cool, add ethanol to make the alcohol content reach 75%, stir well, let it stand for 24 hours, filter, recover ethanol from the filtrate and concentrate to 80°C to a clear paste with a relative density of 1.20-1.25, add appropriate amount of water, Stir evenly, refrigerate at 4-7°C for 36-38 hours, perform ultrafiltration, a...
Embodiment 2
[0037] (1) Preparation of active ingredient: same as Example 1.
[0038] (2) Preparation of solution for atomized inhalation:
[0039]
[0040] Weigh the active ingredient according to the prescription amount, add an appropriate amount of water for injection, and stir evenly to obtain solution 1; take sodium chloride, add appropriate amount of water for injection, stir to dissolve, and obtain solution 2; combine solution 1 and solution 2, and stir evenly to obtain solution 2. Solution 3: Take another appropriate amount of disodium hydrogen phosphate, add an appropriate amount of water for injection to prepare a 0.4mol / L solution, stir slowly and add it to solution 3, adjust the pH value to 4.0-6.0, add an appropriate amount of water for injection, fill and seal, Instantly.
Embodiment 3
[0042] (1) Preparation of active ingredient: same as Example 1.
[0043] (2) Preparation of solution for atomized inhalation:
[0044]
[0045] Weigh the active ingredient according to the prescription amount, add an appropriate amount of water for injection, and stir evenly to obtain solution 1; take magnesium chloride and citric acid, add appropriate amount of water for injection, stir to dissolve, and obtain solution 2; combine solution 1 and solution 2, stir evenly, Obtain solution 3; take another appropriate amount of disodium hydrogen phosphate, add appropriate amount of water for injection to prepare a 0.2mol / L solution, stir slowly and add it to solution 3, adjust the pH value to 5.0-6.0, add appropriate amount of water for injection, fill and seal , that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com