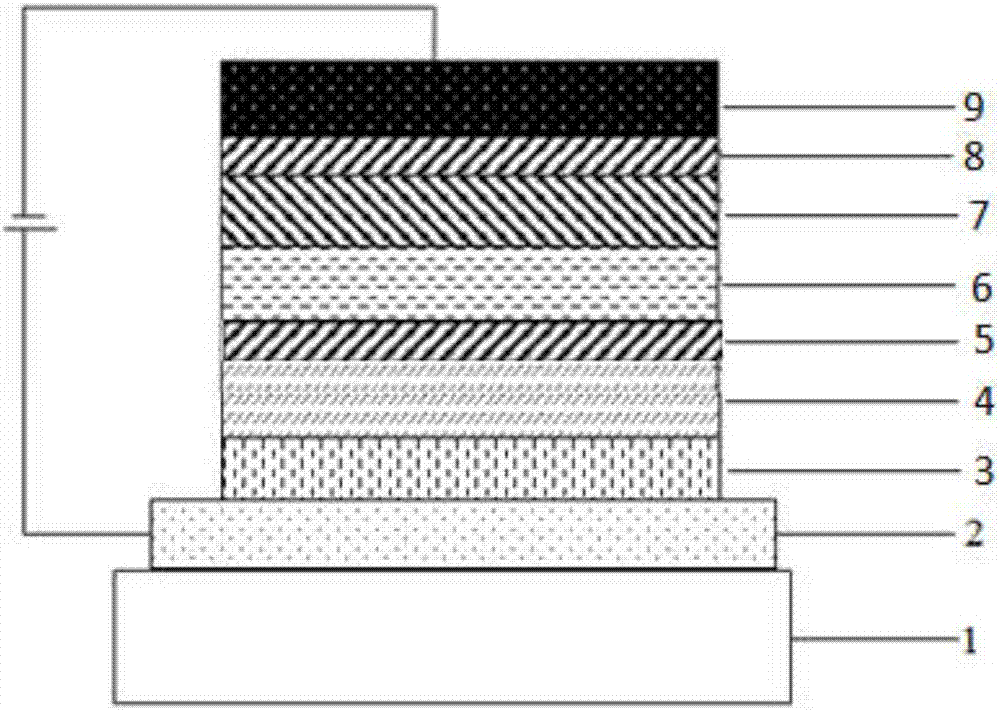
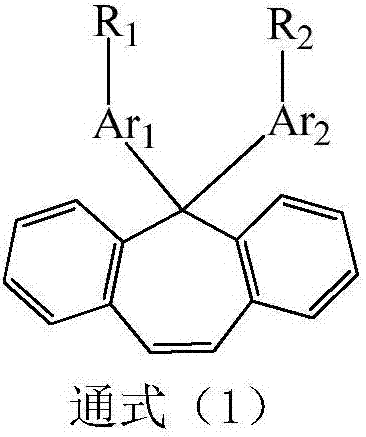
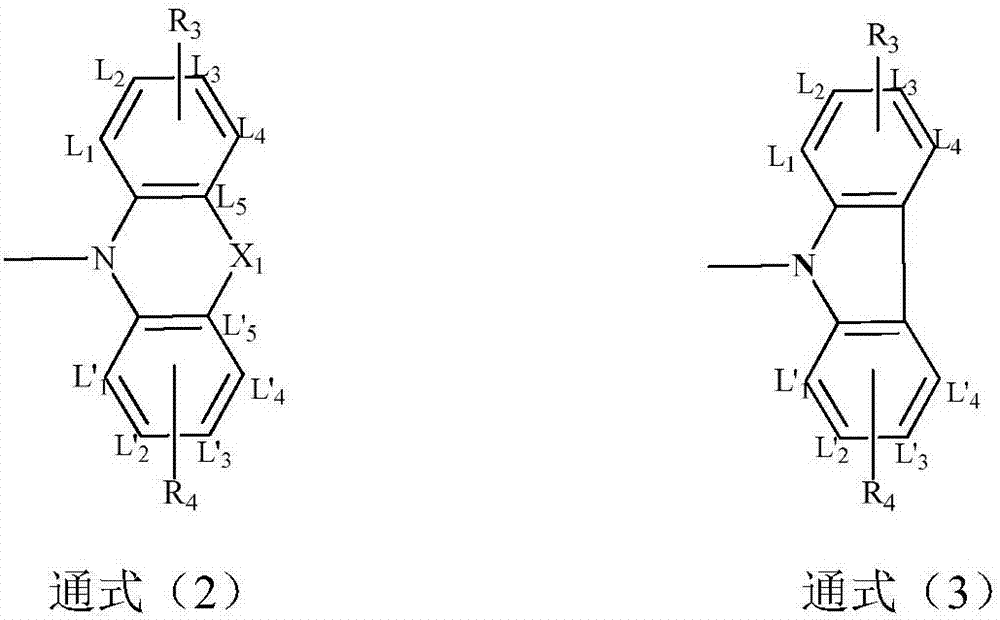
Compound taking diphenyl cycloheptene as core and application thereof in organic light-emitting device
A technology of benzocycloheptene and compounds, applied in the direction of electric solid-state devices, electrical components, luminescent materials, etc., can solve very different problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the synthesis of intermediate A1:
[0067]
[0068] Add 11.8g of 1,4-dibromobenzene (0.05mol) and 1.2g of Mg powder (0.05mol) and 60ml of tetrahydrofuran to a 250ml four-necked flask under nitrogen atmosphere, and heat to reflux for 4 hours. The reaction is complete and the format Reagent;
[0069] Dissolve 10.3g of dibenzocyclopentanone (0.05mol) in 50mL of tetrahydrofuran, add the above-mentioned Grignard reagent dropwise, react at 60°C for 24 hours, a large amount of white Grignard salt precipitates, add saturated NHCl 4 solution until the precipitation disappears, and the Grignard salt is converted into a tertiary alcohol; after the reaction is completed, extract with 100ml ether, dry the extract with anhydrous sodium sulfate, and remove the solvent by rotary evaporation until there is no fraction to obtain the crude product of the tertiary alcohol, which is obtained as The mixed solvent of petroleum ether and dichloromethane (volume ratio 3:2) was...
Embodiment 2
[0071] Embodiment 2: the synthesis of intermediate A2:
[0072]
[0073] Prepare intermediate A2 according to the synthesis method of intermediate A1 in Example 1, the difference is that 1,1'-biphenyl is used to replace the compound bromobenzene; use DEI-MS to identify the compound, molecular formula C 33 h 23 Br, detection value [M+1] + =500.31, calculated value 499.44.
Embodiment 3
[0074] Embodiment 3: the synthesis of intermediate A3:
[0075]
[0076] Prepare intermediate A3 according to the synthetic method of intermediate A1 in Example 1, the difference is that benzene is used instead of bromobenzene in the third step reaction; use DEI-MS to identify the compound, molecular formula C 27 h 19 Br, detection value [M+1] + =424.21, calculated value 423.34.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com