Reversible hydrogen bond self-healing polymer and preparation method thereof
A self-healing, polymer technology, applied in the preparation of organic compounds, preparation of urea derivatives, chemical instruments and methods, etc., can solve the problems of difficult to realize industrial production, complex synthesis operation of UPy linking unit, high production cost, etc. Simple and easy to handle, realize industrialized operation, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
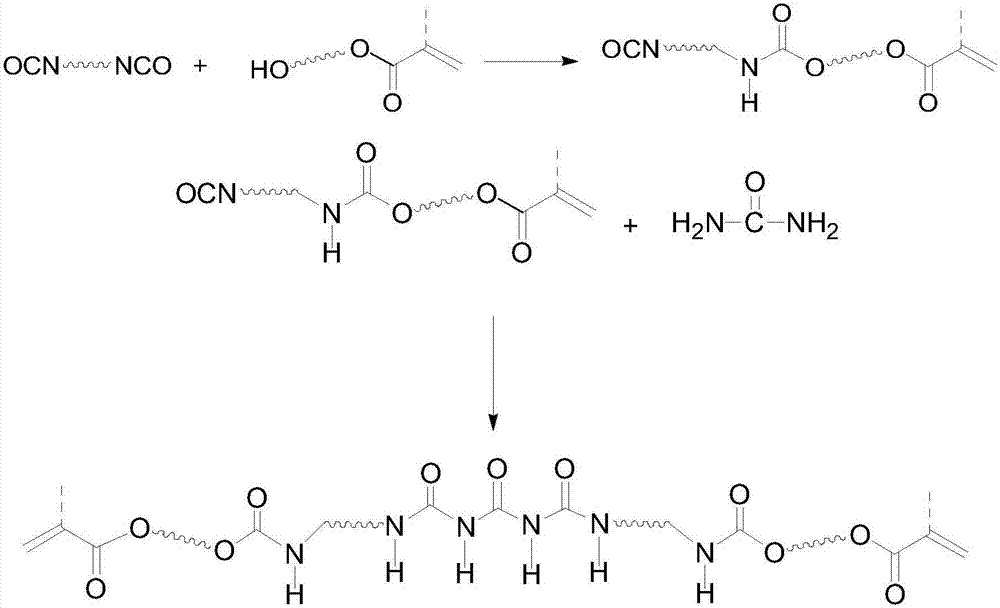
[0022] (1) At room temperature, add 0.3mol isophorone diisocyanate (IPDI), 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol into a dry reaction flask, Add 0.3mol hydroxyethyl acrylate (HEA) dropwise under stirring, raise the temperature to 40-50°C after the dropwise addition, and stir the reaction until the measured -NCO value reaches half of that at the beginning of the reaction, that is, the semi-blocked isocyanate IPDI is prepared -HEA monomer;
[0023] (2) Keep the temperature of the reaction system, add 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol to the reaction flask of step (1), batch by batch under stirring Add 0.15 mol of urea, then raise the temperature to 60-70°C, and stir the reaction until the measured isocyanate group (-NCO) value reaches the theoretical value, that is, the reversible hydrogen bond self-healing polymer is prepared.
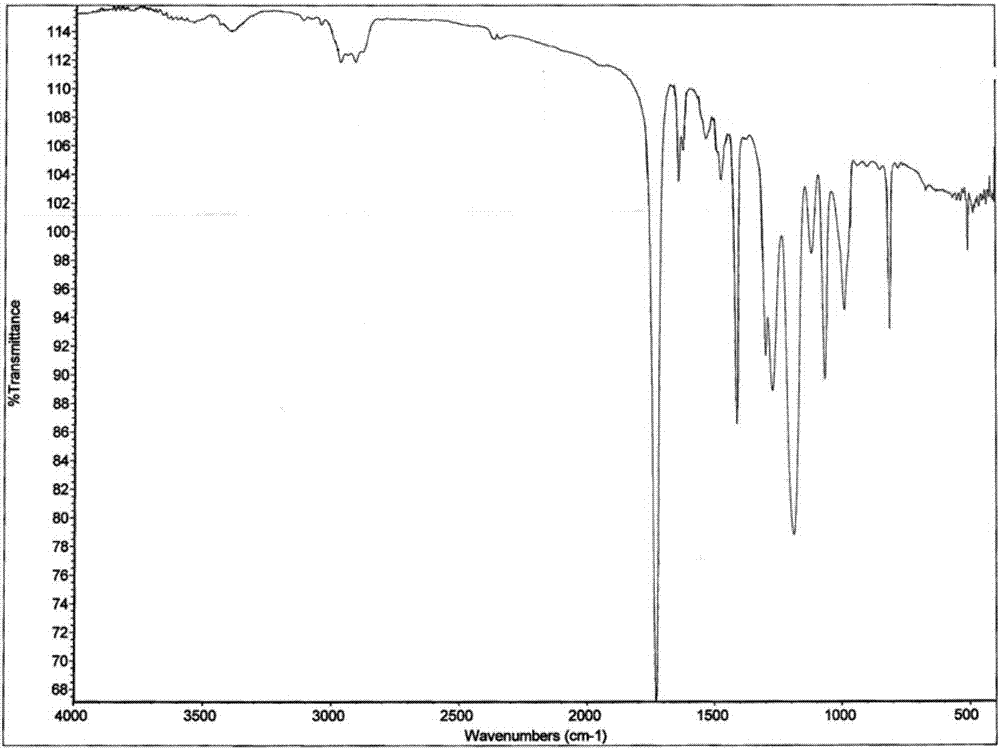
[0024] Synthetic route such as figure 1 As shown, the infrared spectrum of the obtained self-h...
Embodiment 2
[0026] (1) At room temperature, add 0.2mol hexamethylene diisocyanate (HDI), 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol into a dry reaction flask, Add 0.2mol polycaprolactone acrylate (CA) dropwise under stirring, raise the temperature to 40-50°C after the dropwise addition, and stir the reaction until the measured -NCO value reaches half of that at the beginning of the reaction, that is, the half-capped Isocyanate HDI-CA monomer;
[0027] (2) Keep the temperature of the reaction system, add 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol to the reaction flask of step (1), batch by batch under stirring Add 0.1 mol of urea, then raise the temperature to 60-70°C, and stir the reaction until the measured isocyanate group (-NCO) value reaches the theoretical value, that is, the reversible hydrogen bond self-healing polymer is prepared.
Embodiment 3
[0029] (1) At room temperature, add 0.21mol diphenylmethane diisocyanate (MDI), 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol into a dry reaction flask, Add 0.21mol hydroxyethyl methacrylate (HEMA) dropwise under stirring, raise the temperature to 40-50°C after the dropwise addition, and stir the reaction until the measured -NCO value reaches half of that at the beginning of the reaction, that is, the semi-blocked Isocyanate MDI-HEMA monomer;
[0030] (2) Keep the temperature of the reaction system, add 0.05wt% dibutyltin dilaurate, 0.2wt% 2,6-di-tert-butyl p-cresol to the reaction flask of step (1), batch by batch under stirring Add 0.1 mol of urea, then raise the temperature to 60-70°C, and stir the reaction until the measured isocyanate group (-NCO) value reaches the theoretical value, that is, the reversible hydrogen bond self-healing polymer is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com