Method for removing H2 impurity from CO raw gas through selective oxidation of NO
A technology of selective oxidation and raw material gas, applied in chemical instruments and methods, carbon monoxide, separation methods, etc., can solve problems such as excess nitrite, decomposition, etc., and achieve the effect of simplifying the process and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
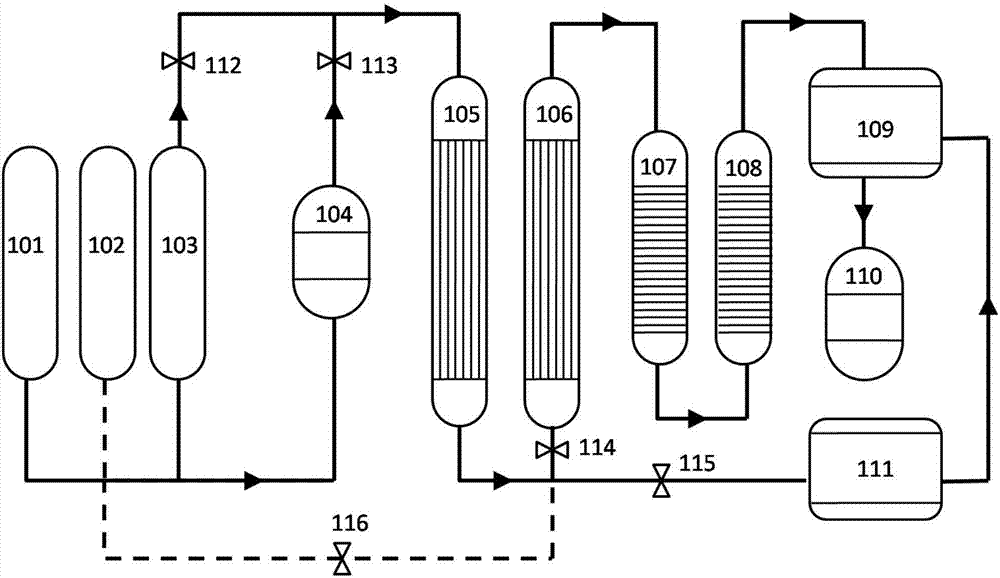
[0022] 1. Weigh 5g catalyst I Pd / CaO-Nb 2 o 5 Dilute it with quartz sand at 1:4 and fill it into fixed bed I (105). The mass percent of Pd in catalyst I is 1wt.%, the mass percent of CaO is 40wt.%, and the mass percent of Nb2 o 5 The mass percentage of is 59wt.%. Weigh 5g Catalyst II Pd-Rh / CeO 2 Dilute with quartz sand at a ratio of 1:1 and fill it into fixed bed II (106). The mass percentage of Pd in catalyst II is 1.5wt.%, the mass percentage of Rh is 0.3wt.%, CeO 2 The mass percentage of is 98.2wt.%.
[0023] 2. Open 112 and close 113, 114 and 115 to switch the system to the pretreatment gas circuit. The fixed bed I (105) is preheated to 80°C and then directly fed with NO gas. When the pressure reaches 0.5MPa, the 112 is closed, and the 115 is opened to vent after 0.5h of treatment. After the pressure drops to 0.1 MPa, close 115 and open 112 again, and repeat the above process 6 times to complete the NO pre-adsorption treatment.
[0024] 3. Close 112 and 115, open...
Embodiment 2
[0029] 1. Weigh 5g catalyst I Rh / MgO-Nb 2 o 5 Dilute it with quartz sand at 1:4 and fill it into fixed bed I (105). The mass percent of Rh in catalyst I is 0.5wt.%, the mass percent of MgO is 30wt.%, and the mass percent of Nb 2 o 5 The mass percentage of is 69.5wt.%. Weigh 5g Catalyst II Pd-Rh / CeO 2 Dilute with quartz sand at 1:1 and pack into fixed bed II (106), the mass percent of Pd in the catalyst II is 1.2wt.%, the mass percent of Rh is: 0.8wt.%, CeO 2 The mass percentage of is 98wt.%.
[0030] 2. Open 112 and close 113, 114 and 115 to switch the system to the pretreatment gas circuit. The fixed bed I (105) is preheated to 80°C and then directly fed with NO gas. When the pressure reaches 0.5MPa, the 112 is closed, and the 115 is opened to vent after 0.5h of treatment. After the pressure drops to 0.1 MPa, close 115 and open 112 again, and repeat the above process 6 times to complete the NO pre-adsorption treatment.
[0031] 3. Close 112 and 115, open 113 and 114....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com