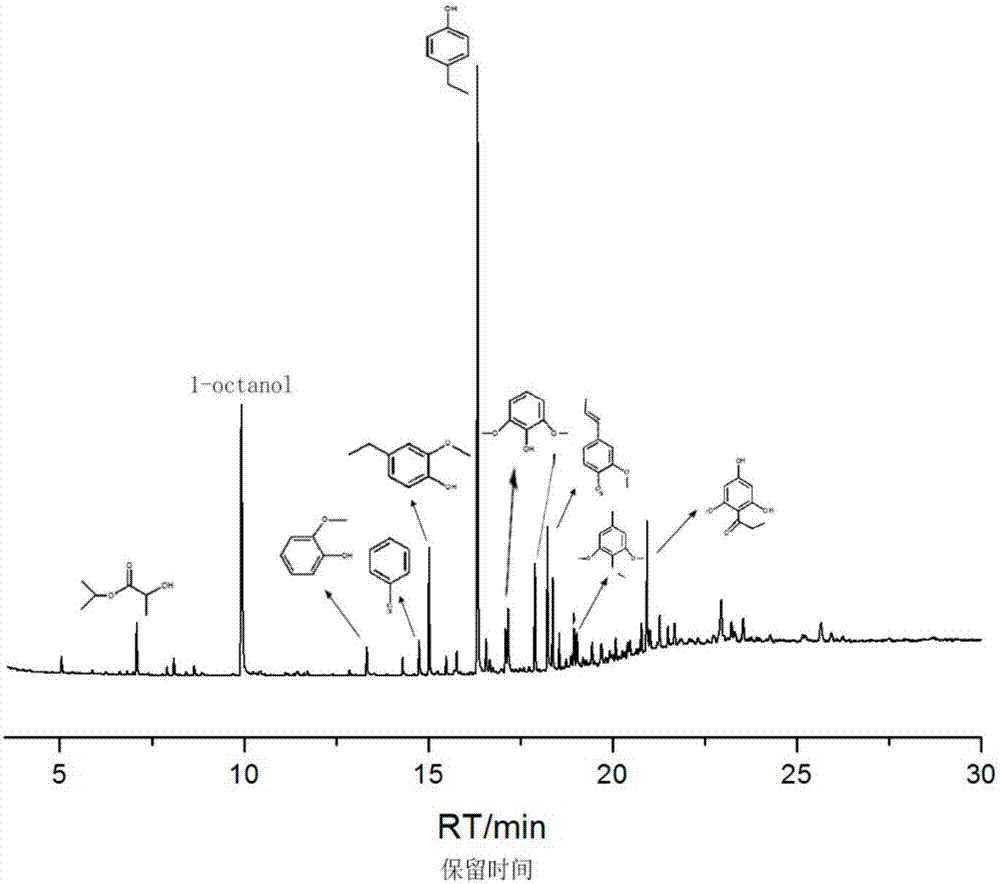
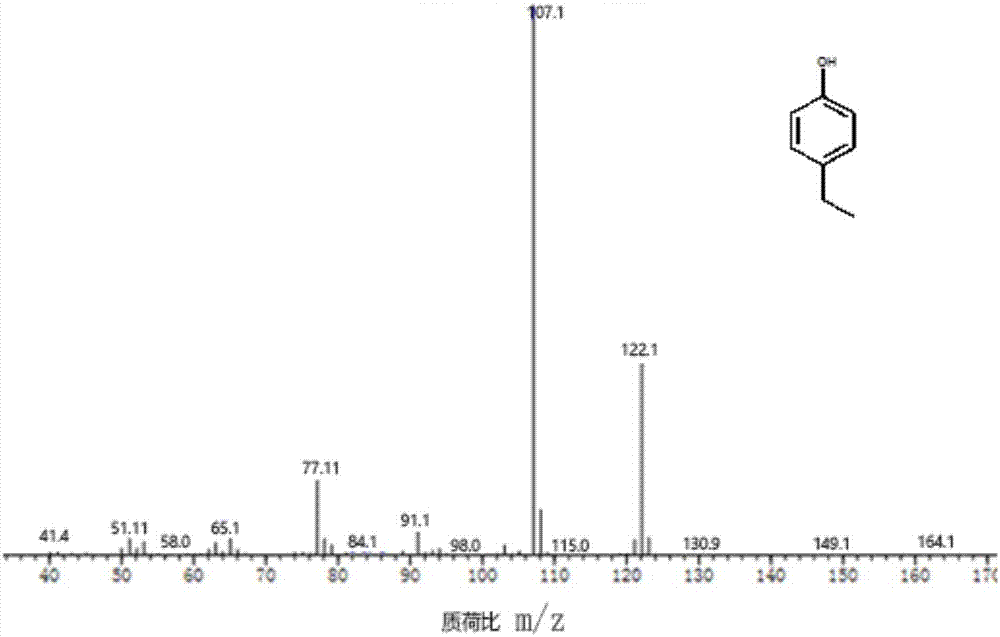
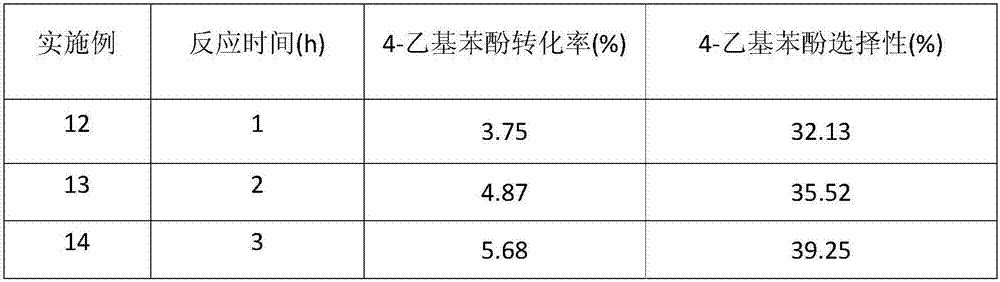
Method for preparing 4-ethyl phenol by selective lignin hydrogenolysis
A ‐ethylphenol and lignin technology, applied in the field of biomass energy utilization, can solve the problem of high cost of precious metals, and achieve the effect of simple preparation method, wide source and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The method for preparing 4-ethylphenol by selective catalytic conversion of lignin comprises the steps:
[0028] Weigh 203.3g of MgCl with a purity of 99% 2 ·6H 2 The solid of O was added to a 500ml beaker, fully dissolved with 200ml of deionized water, then poured into a 1000ml round bottom flask, and 100ml (25%) of NH 3 ·H 2 O while stirring vigorously. A white precipitate was formed and aged for 12 hours, filtered, and the filtrate was treated with Ag(NO 3 ) 2 The solution was detected, and the solid part was carefully washed several times with deionized water until no obvious precipitation was formed. The solid part was placed in an oven at 120°C for one night, and then calcined in a muffle furnace at 850°C for 4 hours to obtain a white powder that is the catalyst carrier MgO.
[0029] Weigh 1.818g of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 2.25ml of deionized water, and after being completely dissolved, it was added to 1g of the prepared carrier MgO. After s...
Embodiment 2
[0040] Take by weighing 339.2g purity and be 99% Zr(NO 3 ) 4 ·5H 2 The solid of O was added to a 500ml beaker, fully dissolved with 200ml of deionized water, then poured into a 1000ml round bottom flask, and 100ml (25%) of NH 3 ·H 2 O while stirring vigorously. A white precipitate was formed and aged for 12 hours, filtered, and the filtrate was treated with Ag(NO 3 ) 2 The solution was detected, and the solid part was carefully washed several times with deionized water until no obvious precipitation was formed. Take the solid part and place it in an oven at 120°C for one night, and then calcinate it in a muffle furnace at 850°C for 4 hours to obtain a white powder that is the catalyst carrier ZrO 2 .
[0041] Weigh 1.818g of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 2.25ml of deionized water, and after it was completely dissolved, it was added to 1g of carrier ZrO prepared in step 1). 2 middle. After soaking for 12 hours, dry it in a drying oven at 110°C for 12 hours, a...
Embodiment 3
[0045] Weigh 101.7g of MgCl with a purity of 99% 2 ·6H 2 The solid of O was added to a 500ml beaker, fully dissolved with 200ml of deionized water, then poured into a 1000ml round bottom flask, and 72ml (25%) of NH 3 ·H 2 O while stirring vigorously. A white precipitate was formed and aged for 12 hours, filtered, and the filtrate was treated with Ag(NO 3 ) 2 The solution was detected, and the solid part was carefully washed several times with deionized water until no obvious precipitation was formed. The solid part was placed in an oven at 120°C for one night, and then calcined in a muffle furnace at 850°C for 4 hours to obtain a white powder that is the catalyst carrier MgO.
[0046] Weigh 1.818g of Ni(NO 3 ) 2 ·6H 2 O was dissolved in 2.25ml of deionized water, and after being completely dissolved, it was added to 1g of the prepared carrier MgO. After soaking for 24 hours, dry it in a 110°C drying oven for 18 hours, and finally calcinate the dried solid in a muffle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com