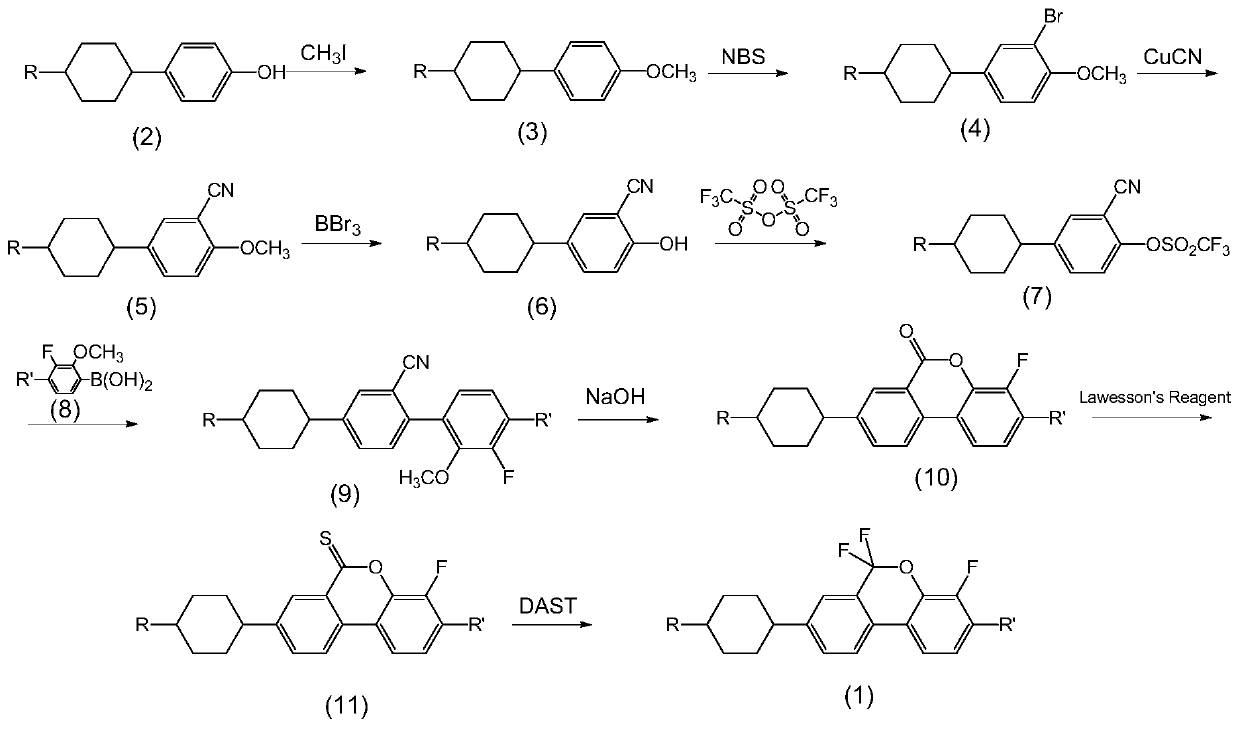
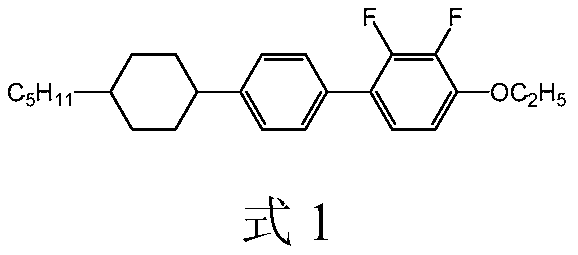
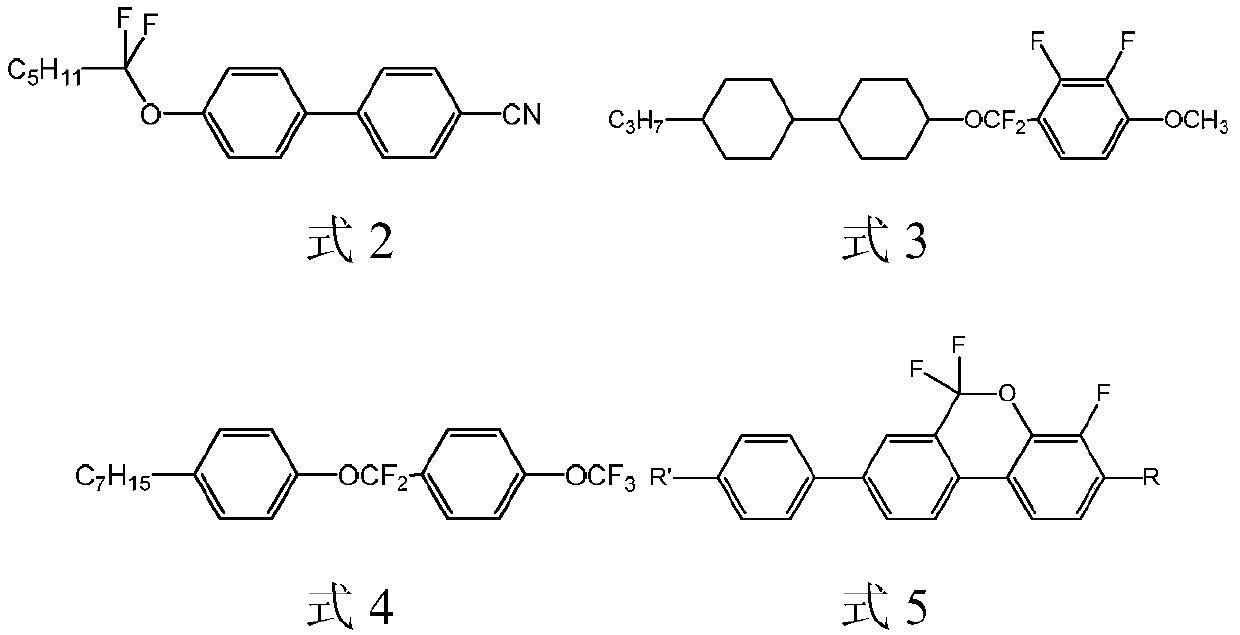
Lateral difluoromethylene ether bridge double-terminal alkylcyclohexylbiphenyl derivatives and preparation method and application thereof
A technology of alkyl ring and hexyl, which is applied in the field of side difluoromethylene ether bridge double-terminal alkyl cyclohexyl biphenyl derivatives and its preparation and application, which can solve the problem of large conjugated system and optical anisotropy Big, limited, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] The preparation of embodiment 1,1-methoxy group-4-(4-pentylcyclohexyl) benzene
[0164] 250mL three-necked bottle, equipped with mechanical stirring, thermometer, reflux condenser, put 4.92g (20.0mmol) 4-(4-pentylcyclohexyl) phenol, 2.84g (20.0mmol) CH 3 I, 2.76g (20.0mmol) K 2 CO 3 , 70mL of acetone, reflux (temperature is 56 ℃) for 6 hours, stop the reaction. The solid was removed by filtration, and the solvent was evaporated by a rotary evaporator, and the obtained crude product was purified by silica gel column chromatography, and the eluent was petroleum ether. The eluent was evaporated to remove the solvent with a rotary evaporator to obtain 4.80 g of a crude solid product. The crude product was recrystallized from petroleum ether to obtain 3.50 g of white crystals (GC purity 99%), with a yield of 65%.
[0165] Structural Confirmation Data:
[0166] IR(KBr)ν max / cm -1 :3035, 3001, 2954, 2916, 2846, 1614, 1514, 1248, 1036;
[0167] 1 HNMR (300MHz) δ: 7.12...
Embodiment 2
[0179] The preparation of embodiment 2,1-methoxy-2-bromo-4-(4-pentylcyclohexyl)benzene
[0180] 2L three-necked flask, equipped with a mechanical stirrer and a thermometer, put in 36.00g (138.0mmol) 1-methoxy-4-(4-pentylcyclohexyl)benzene, 1000mL acetonitrile, 10°C, and stir until all solids in the reaction solution are dissolved , added 24.60g (138.0mmol) NBS in batches, and reacted for another 7 hours after the addition was completed. The solvent was evaporated by a rotary evaporator, and the obtained crude product was purified by silica gel column chromatography, and the eluent was petroleum ether. The eluent was evaporated to remove the solvent with a rotary evaporator to obtain 53.00 g of crude product. The crude product was recrystallized from petroleum ether to obtain 42.00 g of white crystals (GC purity 96%), with a yield of 89%.
[0181] Structural Confirmation Data:
[0182] IR(KBr)ν max / cm -1 : 3032, 3007, 2953, 2918, 2848, 1257, 1055;
[0183] 1 HNMR (300MHz)...
Embodiment 3
[0196] The preparation of embodiment 3,1-methoxy-2-cyano-4-(4-pentylcyclohexyl)benzene
[0197] 250mL three-necked flask, equipped with mechanical stirring, thermometer, reflux condenser, put 2.78g (8.2mmol) 1-methoxy-2-bromo-4-(4-pentylcyclohexyl)benzene, 1.80g (20.0mmol) CuCN, 70mL N-methylpyrrolidone, reflux (at 203°C) for 6 hours, cool down to 60°C, add 2.00g FeCl 3 , 0.5mL HCl, 20mL water, stirred for 1 hour. The reaction solution was cooled to room temperature, extracted with chloroform (30 mL×3), and the organic phase was washed with water until neutral. After removing the solvent with a rotary evaporator, 2.50 g of crude product was obtained. The obtained crude product was purified by silica gel column chromatography, and the eluent was a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:10. The eluent was evaporated to remove the solvent with a rotary evaporator to obtain 2.00 g of crude product. The crude product was recrystallized with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com