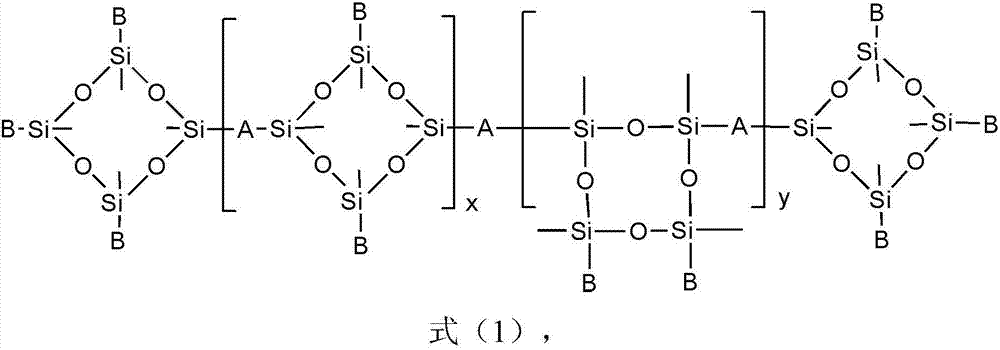
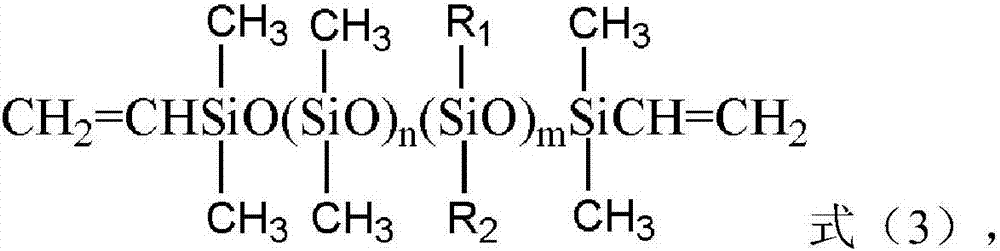
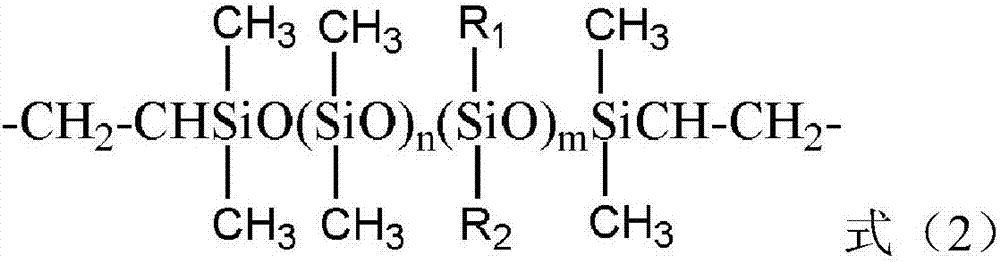Preparation and application of polycyclic siloxane polyether copolymer
A technology based on siloxane copolymer and polysiloxane, which is used in skin care preparations, cosmetic preparations, cosmetic preparations, etc., can solve problems such as no research reports, and achieve effective antistatic regulation, easy Effective control of emulsifying properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a four-neck flask equipped with a constant-pressure dropping funnel, a reflux condenser, a thermometer, and an electric stirrer, weigh 103.68g (0.12mol) in turn according to the metering ratio. 2 =CHCH 2 (OC 2 h 4 ) 18 OCH 3 (a=18, b=0, R=-CH 3 ) of methoxy-terminated allyl polyoxyethylene ether (APER, average molecular weight M n =864) and 1.86g (0.01mol) α, ω-bisvinyltetramethyldisiloxane (VPS, n=0, m=0, Mn186.4), (D 4 H :APER:VPS=2:6:1), and raw material (D 4 H +APER+VPS) total mass (110.62g) 10%, that is, 11.06g ethanol solvent, stir and mix evenly, inert gas protection, and weigh 4.96g (0.02mol) 1,3,5,7-tetramethylcyclo Tetrasiloxane (D 4 H , M n =248) into the constant pressure dropping funnel, heated up to 80°C, added the calculated amount of chloroplatinic acid catalyst to the four-necked flask, so that the platinum content in the system was 3ppm, then opened the constant pressure dropping funnel to remove D 4 HSlowly add it into a four-necked f...
Embodiment 2
[0032] In a four-neck flask equipped with a constant-pressure dropping funnel, a reflux condenser, a thermometer, and an electric stirrer, weigh 66.13g (0.022mol) in sequence according to the metering ratio. 2 =CHCH 2 (OC 2 h 4 ) 42 (OC 3 h 6 ) 18 OC 4 h 9 (APER, a=42, b=18, R=-C 4 h 9 ) of butoxy-terminated allyl polyoxyethylene polyoxypropylene ether (average molecular weight M n =3006) and 1.68g (0.009mol) α, ω-vinyl-terminated tetramethyldisiloxane (VPS, n=0, m=0, M n =186.4), (D 4 H :APER:VPS=1.0:2.2:0.9), and raw material (D 4 H +APER+VPS) total mass (70.31g) 100% 70.31g isopropanol solvent, stir and mix evenly, inert gas protection, and weigh 2.48g (0.01mol) 1,3,5,7-tetramethylcyclo Tetrasiloxane (D 4 H , M n =248) into the constant pressure dropping funnel, heated up to 80°C, added the calculated amount of chloroplatinic acid catalyst to the four-necked flask, so that the platinum content in the system was 25ppm, then opened the constant pressure drop...
Embodiment 3
[0035] In a four-neck flask equipped with a constant pressure dropping funnel, a reflux condenser, a thermometer, and an electric stirrer, 56.41g (0.036mol) of CH 2 =CHCH 2 (OC 2 h 4 ) 30 (OC 3 h 6 ) 3 OCH 3 (APER, a=30, b=3, R=-CH 3 ) of methoxy-terminated allyl polyoxyethylene polyoxypropylene ether (average molecular weight M n =1567 and 9.34g (0.012mol) α, ω-divinyl terminated polydimethylsiloxane (VPS, n=8, m=0, M n =778.4), (D 4 H :APER:VPS-8=5:12:4), and raw material (D 4 H +APER+VPS) total mass (69.47g) 50% of 34.74g isopropanol-propylene glycol butyl ether (both mass ratio is 50:50) mixed solvent, stirring and mixing, inert gas protection, and weighed 3.72g ( 0.015mol) 1,3,5,7-tetramethylcyclotetrasiloxane (D 4 H , M n =248) into the constant pressure dropping funnel, then heated up to 85°C, added the calculated amount of complex platinum catalyst to the four-necked flask, so that the platinum content in the system was 50ppm, then opened the constant p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com