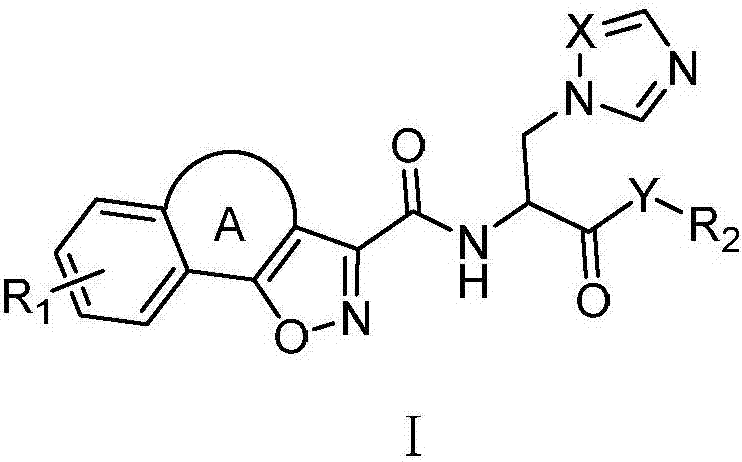
Tricyclic isoxazole derivative, preparation method thereof and application
A technology of isoxazoles and derivatives, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as drug resistance, narrow antibacterial spectrum, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
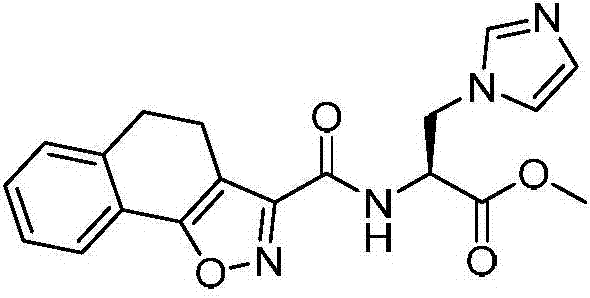
[0083] Example 1: Preparation of (S)-2-(4,5-dihydronaphthoisoxazole-2-carboxamido)-3-(1H-imidazol-1-yl)propionic acid methyl ester
[0084]
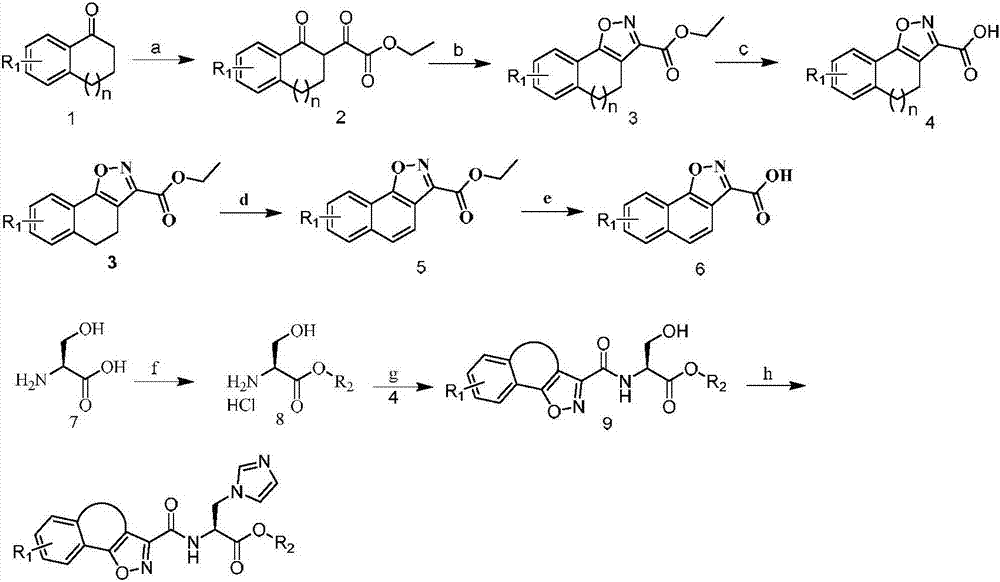
[0085] The synthetic route is as follows:
[0086]
[0087] Reagents and conditions: a) diethyl oxalate, LiHMDS, tetrahydrofuran; b) hydroxylamine hydrochloride, glacial acetic acid; c) 1N sodium hydroxide, ethanol; d) thionyl chloride, methanol; e) EDCI, HOBt, DMF; f ) CDI, imidazole, acetonitrile.
[0088] 3.0g (20.5mmol) of 1-tetralone and 3.6g (24.6mmol) of diethyl oxalate were dissolved in 60mL of tetrahydrofuran, and LiHMDS solution (1M in THF,) was slowly added dropwise under ice-bath conditions. After reacting at room temperature for 4 h, TLC detected that the reaction was complete, and the reaction solution was evaporated to dryness and used directly for the next reaction.
[0089] The concentrate from the previous step was dissolved in 60 mL of glacial acetic acid, 1.70 g (24.6 mmol) of hydroxylamine hydrochloride was add...
Embodiment 2
[0095] Example 2: Preparation of (S)-2-(4,5-dihydronaphthoisoxazole-2-carboxamido)-3-(1H-imidazol-1-yl)propionic acid isopropyl ester
[0096]
[0097] 1 H NMR (600MHz, DMSO-d 6 )δ9.23(d, J=8.1Hz, 1H), 7.67(t, J=8.4Hz, 1H), 7.62(s, 1H), 7.39(ddd, J=10.3, 7.3, 2.1Hz, 3H), 7.20(s,1H),6.85(s,1H),4.96(m,1H),4.82(m,1H),4.51(dd,J=14.1,5.0Hz,1H),4.41(dd,J=14.1, 9.7Hz, 1H), 3.01(t, J=7.9Hz, 2H), 2.89–2.79(m, 2H), 1.20(t, J=6.5Hz, 6H). 13 C NMR (100MHz, DMSO-d 6 )δ168.43, 166.47, 159.34, 154.86, 137.78, 136.86, 130.52, 128.79, 128.00, 127.24, 123.80, 121.47, 119.99, 113.12, 68.97, 53.26, 45.68, 27.472, 27
Embodiment 3
[0098] Example 3: (S)-2-(4,5-dihydronaphthoisoxazole-2-carboxamido)-3-(1H-imidazol-1-yl)propionic acid isobutyl ester
[0099]
[0100] 1 H NMR (600MHz, DMSO-d 6 )δ9.29(d,J=8.2Hz,1H),7.66(d,J=7.3Hz,1H),7.63(s,1H),7.40(ddd,J=13.3,6.9,3.1Hz,3H), 7.21(s,1H),6.86(s,1H),4.90(ddd,J=10.0,8.3,4.8Hz,1H),4.55(dd,J=14.1,4.7Hz,1H),4.44(dd,J= 14.1,10.0Hz,1H),3.91(m,2H),3.01(t,J=7.9Hz,2H),2.83(dd,J=16.7,8.1Hz,2H),1.88(m,1H),0.88( dd,J=6.7,1.6Hz,6H). 13 C NMR (100MHz, DMSO-d 6 )δ169.39, 166.94, 159.84, 155.28, 138.28, 137.30, 130.98, 129.24, 128.81, 127.69, 124.25, 121.93, 120.31, 113.56, 71.27, 53.63, 45.95, 18.28, 297
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com