Method for preparing room temperature halogen-enriched CsPbX3 inorganic perovskite nanocrystal
A nanocrystal, inorganic calcium technology, applied in nanotechnology, nano-optics, nanotechnology and other directions, can solve the problem that the ratio of halogen and cation is not easy to adjust, and achieve the effect of high stability and high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
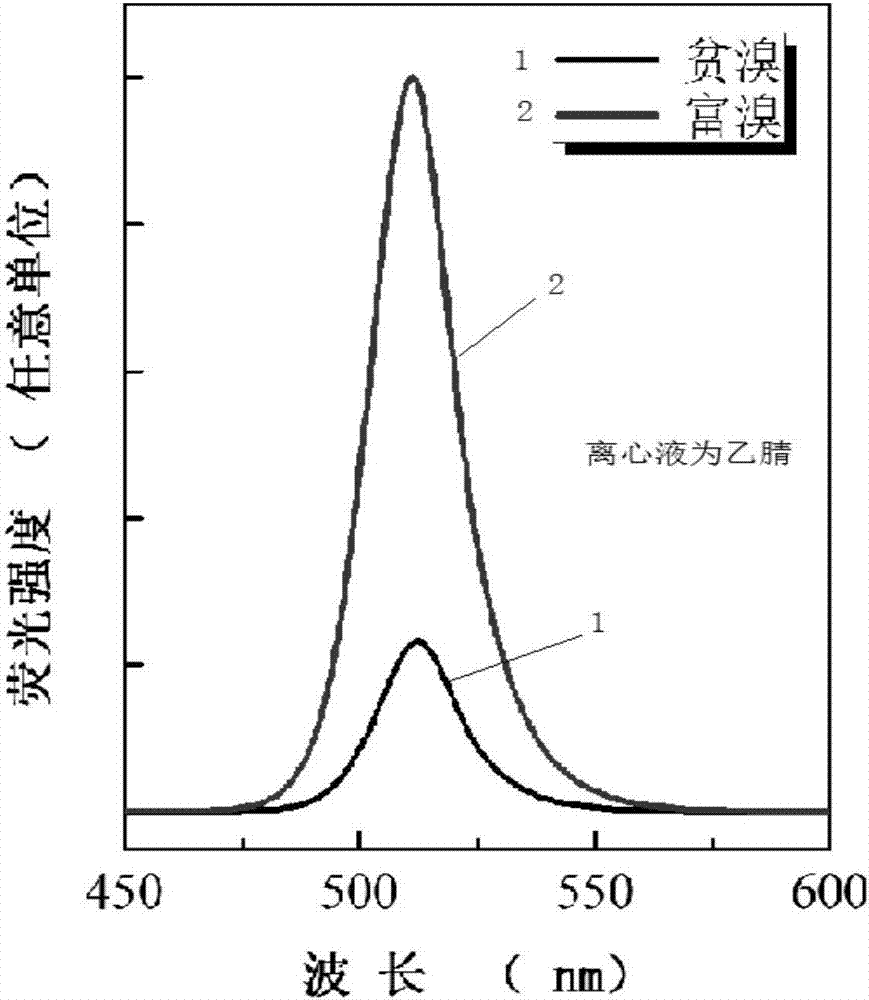
[0024] Described in this embodiment is literature (X.Li, D.Yu, F.Cao, Y.Gu, Y.Wei, Y.Wu, J.Song andH.Zeng, Adv.Funct.Mater., 2016,26 , 2435–2445) published CsPbBr 3 The synthesis method of inorganic perovskite nanocrystals is used as a comparison of the method of the present invention.
[0025] 1. Add 0.4mmol of PbBr 2 and 0.4mmol of CsBr were added to 10ml of DMF, and ultrasonically dissolved completely;
[0026] 2. Add 1ml of oleic acid and 0.5ml of oleylamine as surfactants to the above precursor solution and mix them evenly;
[0027] 3. Take 1ml of the mixed solution and pour it into 10ml of toluene, stir to make it react completely, the stirring speed is 800r / min, then mix the original solution and acetonitrile in a volume ratio of 1:1, and then centrifuge, the centrifugal speed is 8000r / min min, and the centrifugation time was 5 min, and finally the metal halide inorganic perovskite nanocrystals were obtained. See the test results figure 1
Embodiment 2
[0029] The method described in this embodiment is an improved method proposed by the present invention, and the same CsPbBr 3 Inorganic perovskite nanocrystal synthesis as an example.
[0030] 1. Add 0.4mmol of PbBr 2 and 0.4mmol of CsBr were added to 10ml of DMF, and ultrasonically dissolved completely;
[0031] 2. Add 1ml of oleic acid and 0.5ml of ammonium bromide to the above precursor solution and mix them evenly. The preparation method of ammonium bromide is to take an appropriate amount of NH 4 Br and oleylamine were added to a three-necked flask, and degassed under an inert gas atmosphere at 100°C for 1 hour to obtain a bromide solution;
[0032] 3. Take 1ml of the mixed solution and inject it into 10ml of toluene, stir to make the reaction complete, the stirring speed is 800r / min, then mix it with acetonitrile at a ratio of 1:1, and then centrifuge, the centrifugal speed is 8000r / min, the centrifugation time for 5 min, and finally the metal halide inorganic perovsk...
Embodiment 3
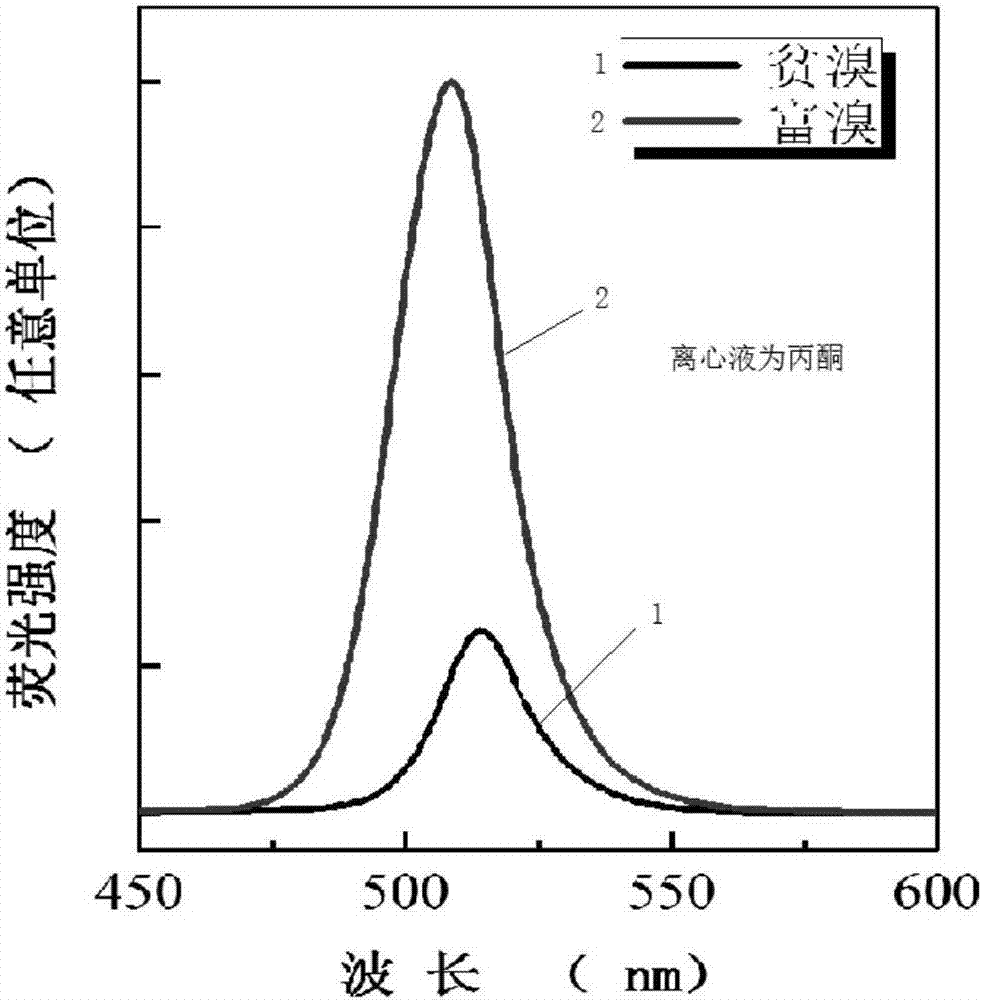
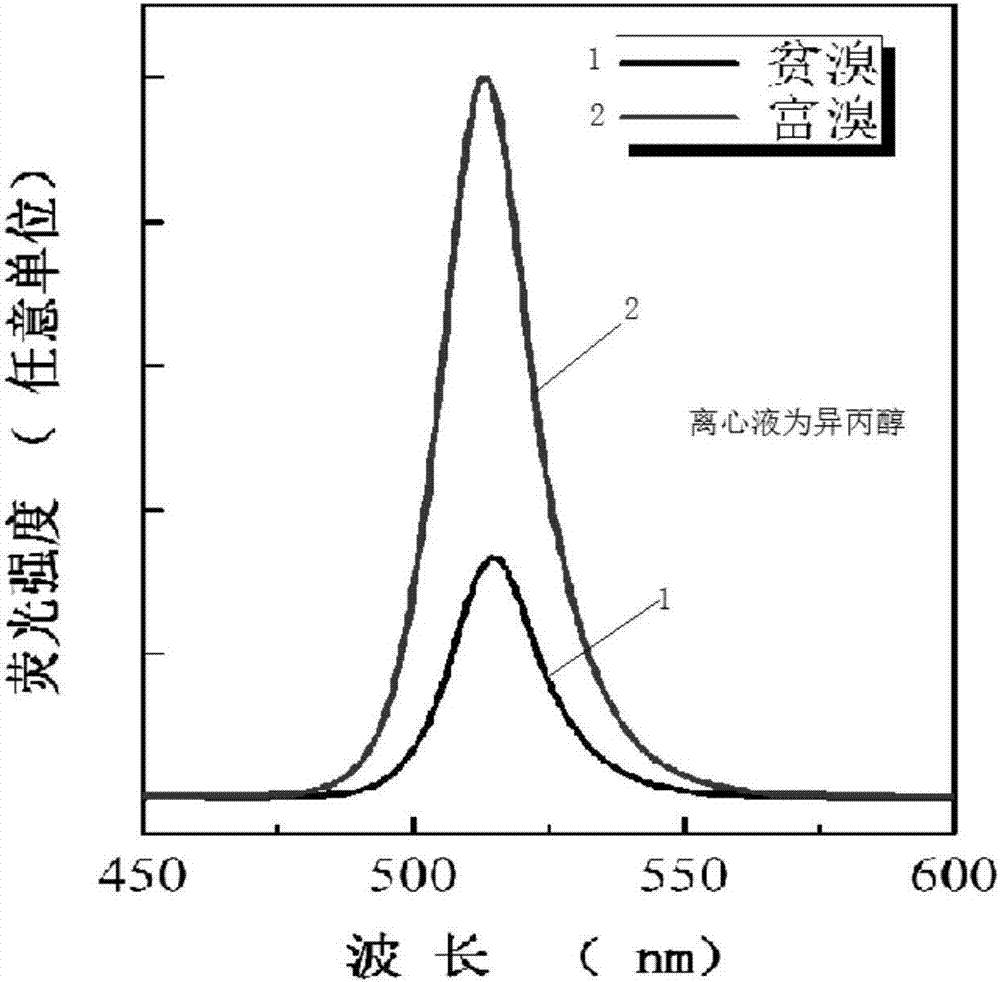
[0036] The present embodiment experiment 3a is identical with embodiment 1, just changes the centrifugal solvent into acetone on the basis of embodiment 1; Present embodiment experiment 3b is identical with embodiment 2, just changes the centrifugal solvent into acetone on the basis of embodiment 2 acetone.
[0037] Such as figure 2 As shown, the fluorescence quantum yield increased by 3 times after centrifugation with acetone to enrich the bromine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com