Ortho-aminophenol Schiff base, and synthesis method and application thereof
A technology of o-aminophenol and a synthesis method, applied in the field of o-aminophenol Schiff base compounds and their synthesis, can solve problems such as poor solubility, and achieve the effects of enhanced fluorescence intensity, good solubility, and increased solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
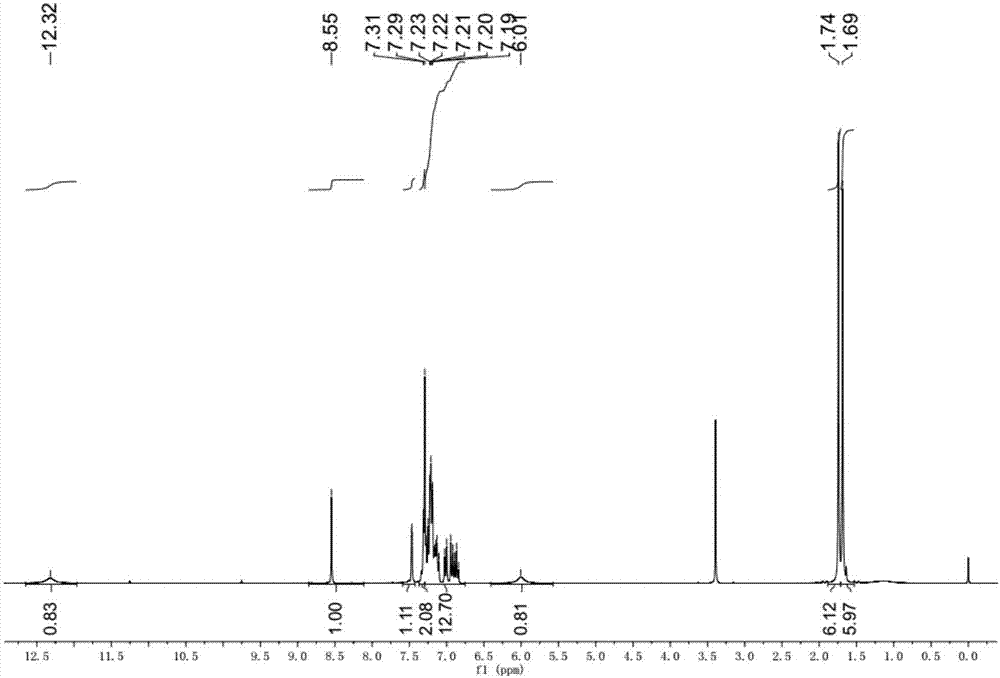
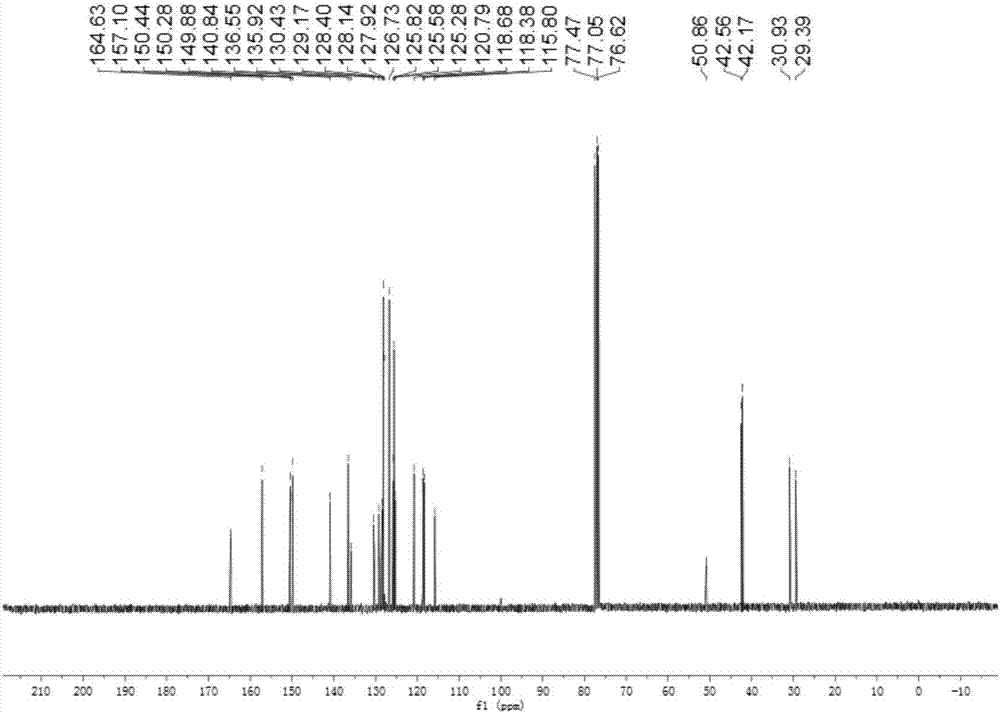
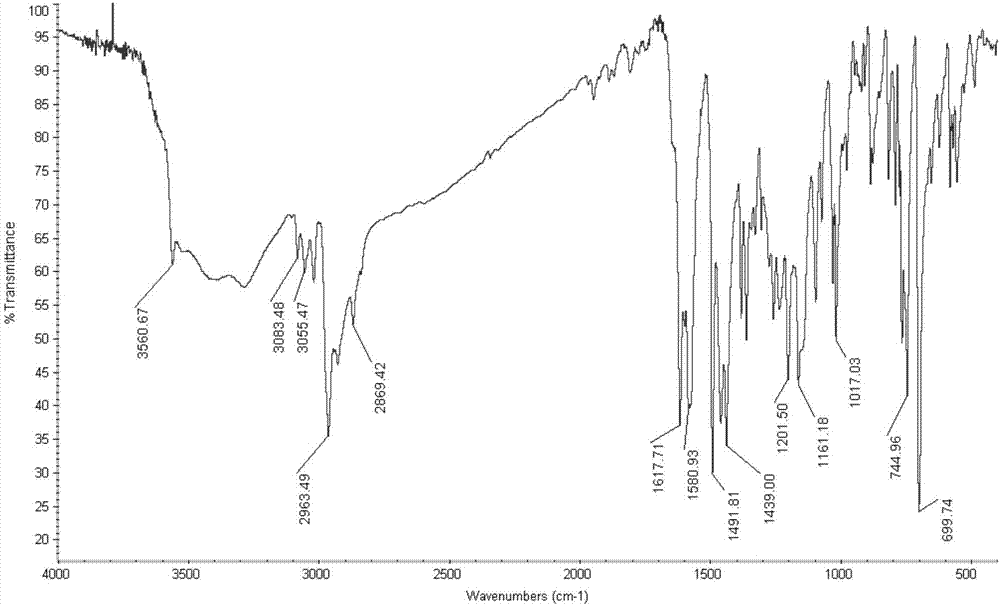
[0049] Specific embodiment one: the structural formula of o-aminophenol Schiff base in this embodiment is as follows:
[0050]
[0051] where R is CH 3 -, tert-octyl or α,α'-dimethyl-α-phenyl.
specific Embodiment approach 2
[0052] Specific embodiment two: the synthetic method of o-aminophenol Schiff base of the present embodiment comprises the following steps:
[0053] 1. Fuckers Alkylation Reaction:
[0054] After mixing the alkylphenol and the catalyst p-toluenesulfonic acid, add α-methylstyrene dropwise, under the protection of nitrogen, heat and reflux, and keep the temperature at 74-76°C. After the dropwise addition, add saturated Na 2 CO3 , adjust the pH to be neutral, concentrate to remove petroleum ether, and collect fractions at 180-200°C by vacuum distillation to obtain intermediate product A;
[0055] Wherein the molar ratio of alkylphenol to catalyst p-toluenesulfonic acid is 1:0.1~1, and the molar weight of α-methylstyrene is 0.9~1 times of the molar weight of alkylphenol;
[0056] Two, formylation reaction:
[0057] After mixing the intermediate product A prepared in step 1 with urotropine, add glacial acetic acid and heat to reflux. When the temperature reaches 120-140°C, keep th...
specific Embodiment approach 3
[0061] Specific embodiment 3: The difference between this embodiment and specific embodiment 2 is that the alkylphenol in step 1 is p-cresol, p-tert-octylphenol or 4-α, α'-dimethyl-α-phenyl phenol. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com