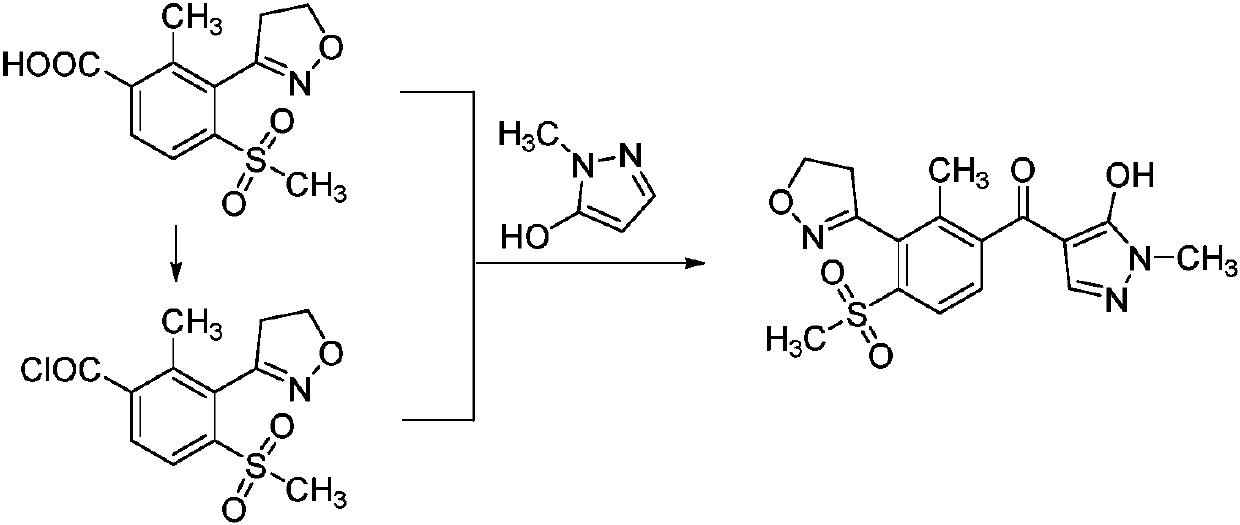
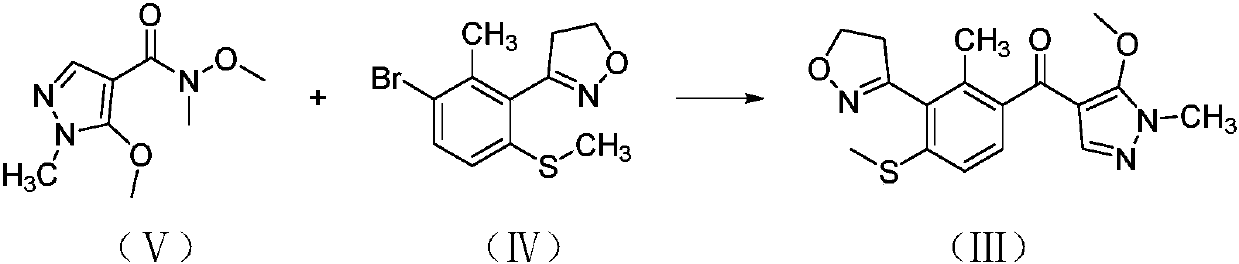
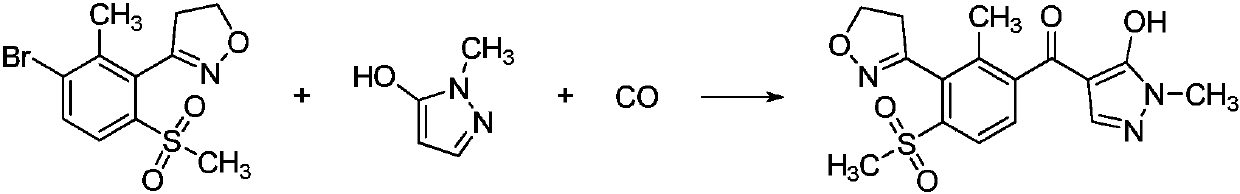A kind of preparation method of fenpyrazone
A technology for toxazone and preparation process, applied in the field of organic compound preparation, can solve the problems of high price, high reaction yield and high cost, and achieve the effects of being beneficial to industrial production, high product yield and realizing localization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of fenpyrazone of the present invention, comprising the following steps:
[0042] (1) Synthesis of compound (VII)
[0043] Add 86.5g (0.4mol) of 2-(ethoxymethylene)diethyl malonate into a three-necked flask, cool to below 30°C, and add 112g (containing 0.8mol of NH 3 ·H 2 O) mass fraction is 25% ammoniacal liquor. After the addition, the temperature of the reaction system was lowered to 5°C, and 25 g (containing 0.4 mol of N 2 h 4 ·H 2 O) The mass fraction is 80% hydrazine hydrate. After the addition is complete, the reaction solution is heated to 60° C. and stirred for 4 hours. After the reaction is completed, cool to below 10° C., and slowly add 81 g (containing 0.8 mol HCl) concentrated hydrochloric acid dropwise to neutralize the aqueous ammonia. It was then extracted with ethyl acetate, dried over anhydrous sodium sulfate, and desolventized to obtain 55.7 g of a light yellow solid with a yield of 89.2%. LC-MS (m / z): 157.1 (M+H + ); 1 H...
Embodiment 2
[0055] A preparation method of fenpyrazone of the present invention, comprising the following steps:
[0056] (1) Synthesis of compound (VII)
[0057] Add 92.1g (0.4mol) of 2-(methoxymethylene)dipropyl malonate into a three-necked flask, cool to below 30°C, and add dropwise 56g (0.4mol) of mass fraction below 30°C under temperature control. 25% ammonia water. After the addition, the temperature of the reaction system was lowered to 0° C., and 37.5 g (0.6 mol) of 80% hydrazine hydrate was added dropwise under heat preservation. After the addition, the temperature of the reaction solution was raised to 80° C. and stirred for 3 hours. After the reaction is completed, cool to below 10° C., and slowly add 40.5 g (containing 0.4 mol HCl) concentrated hydrochloric acid dropwise to neutralize the aqueous ammonia. Then it was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and desolventized to obtain 59.6 g of a light yellow solid with a yield of 87.5%.
[0058] (...
Embodiment 3
[0069] A preparation method of fenpyrazone of the present invention, comprising the following steps:
[0070] (1) Synthesis of compound (VII)
[0071] Add 103.3g (0.4mol) of 2-(methoxymethylene) di-tert-butyl malonate into a three-necked flask, cool to below 30°C, and add 168g (1.2mol) of mass fraction dropwise under temperature control below 30°C 25% ammonia water. After the addition, the temperature of the reaction system was lowered to 5 °C, and 37.5 g (0.6 mol) of 80% hydrazine hydrate was added dropwise under heat preservation. After the addition, the temperature of the reaction solution was raised to 40 °C and stirred for 6 hours. After the reaction was completed, cool to below 10° C., and slowly add 121.5 g (containing 1.2 mol HCl) concentrated hydrochloric acid dropwise to neutralize the aqueous ammonia. It was then extracted with ethyl acetate, dried over anhydrous sodium sulfate, and desolventized to obtain 65 g of a light yellow solid with a yield of 88.2%.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com