Detection method of biological activity of vascular endothelial growth factors and application
A biologically active, vascular endothelial technology, applied in the field of biological product quality detection and analysis, can solve problems affecting the repeatability and reproducibility of alamar blue, and achieve the effect of ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
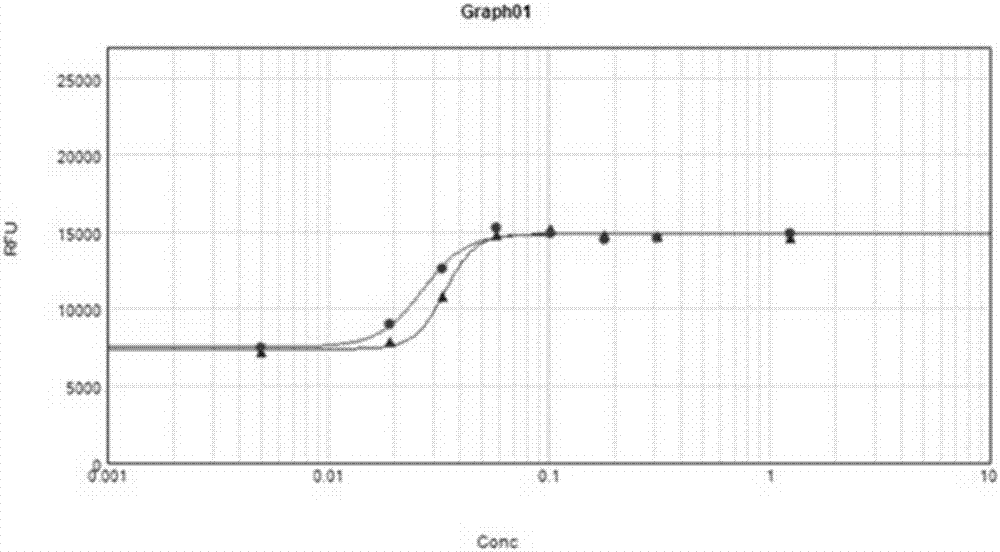
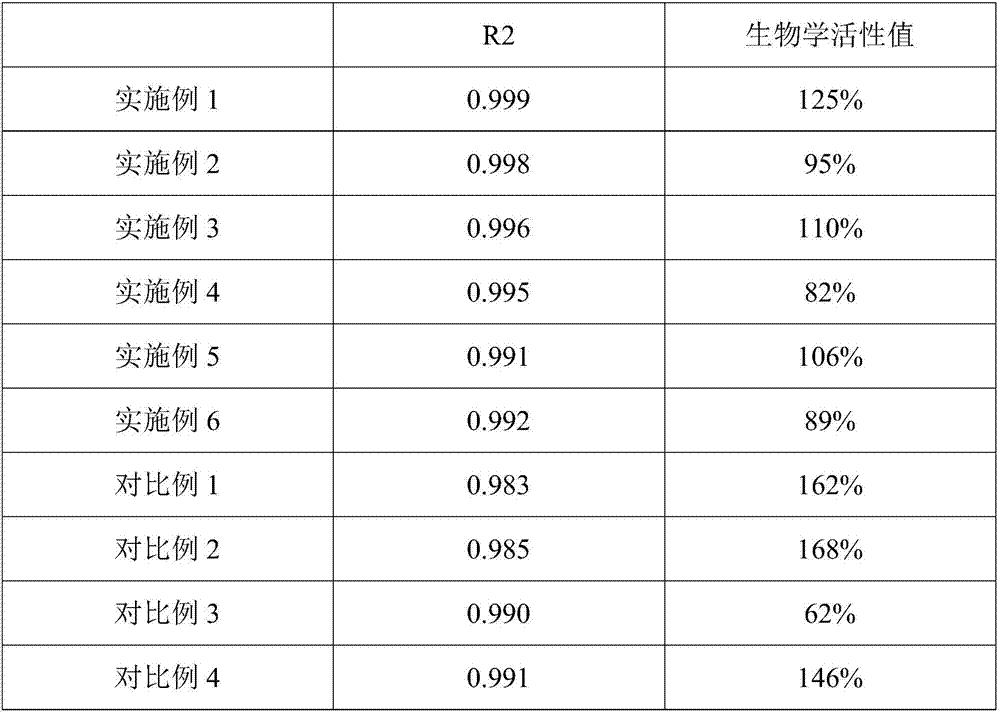
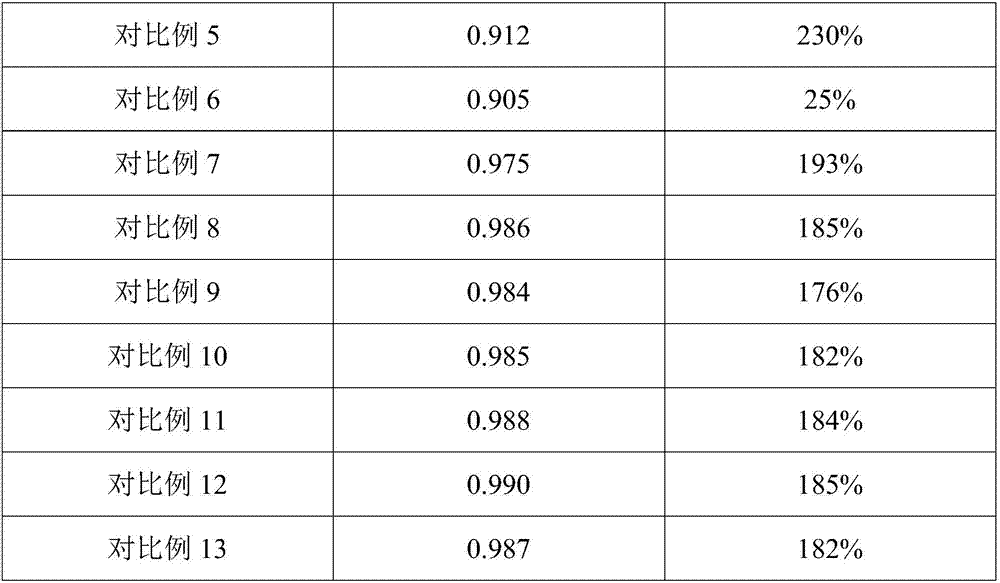
Image
Examples
Embodiment 1
[0078] Quality control is carried out before using different batches of vascular endothelial growth factor purchased, which specifically includes the following steps:
[0079] 1) subpackaging and storing the vascular endothelial growth factor freeze-dried powder;
[0080] The freeze-dried powder of vascular endothelial growth factor was transferred from -20°C to 4°C and equilibrated overnight, and then transferred from 4°C to room temperature for 2 hours, and added to the freeze-dried powder of vascular endothelial growth factor without Bacterial water was dissolved, 25 ° C water bath for 15 min, during which time every 5 min was taken out and inverted to mix, and the dissolved vascular endothelial growth factor was divided into packages, and the tube endothelial growth factor solution was placed at room temperature for 5 min in stages, and then transferred to 4 ° C to balance overnight. Then transfer to -20°C for storage;
[0081] 2) Activity detection of vascular endothelia...
Embodiment 2
[0090] The quality control of vascular endothelial growth factor before use includes the following steps:
[0091] 1) subpackaging and storing the vascular endothelial growth factor freeze-dried powder;
[0092] The freeze-dried powder of vascular endothelial growth factor was transferred from -20°C to 4°C and equilibrated overnight, and then transferred from 4°C to room temperature for 3 hours, and added to the freeze-dried powder of vascular endothelial growth factor without Bacterial water was dissolved, 23 ° C water bath for 16 min, during which every 6 min was taken out and mixed upside down, the dissolved vascular endothelial growth factor was divided into packages, and the tube endothelial growth factor solution was placed at room temperature for 6 min in stages, and then transferred to 4 ° C to balance overnight. Then transfer to -20°C for storage;
[0093] 2) Activity detection of vascular endothelial factor:
[0094] (1) Human umbilical vein endothelial cells were ...
Embodiment 3
[0102] The quality control of vascular endothelial growth factor before use includes the following steps:
[0103] 1) subpackaging and storing the vascular endothelial growth factor freeze-dried powder;
[0104] The freeze-dried powder of vascular endothelial growth factor was transferred from -20°C to 4°C to equilibrate overnight, and then transferred from 4°C to room temperature for 4 hours, and added to the freeze-dried powder of vascular endothelial growth factor Bacterial water was dissolved, 28 ° C water bath for 12 min, during which every 4 min was taken out and mixed upside down, the dissolved vascular endothelial growth factor was divided into packages, and the tube endothelial growth factor solution was placed at room temperature for 6 min in stages, and then transferred to 4 ° C to balance overnight. Then transfer to -20°C for storage;
[0105] 2) Activity detection of vascular endothelial factor:
[0106] (1) Human umbilical vein endothelial cells were cultured t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com