Preparation and application of loaded Pd3Cl cluster catalyst
A catalyst, supported technology, applied in the preparation of organic compounds, physical/chemical process catalysts, carbon-based compounds and other directions, can solve the problems of high reaction temperature, complex catalyst preparation, low yield, etc., to achieve a wide range of applications, Excellent cycle performance, high catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Pd 3 Oxidation of Benzyl Alcohol Catalyzed by Cl@TNT in n-Hexane Solution
[0031] Add 0.5mmol benzyl alcohol and 30mg catalyst Pd to a 10mL Schlenk reaction flask 3 Cl@TNT (Pd load is 1.5wt%) and 1mL n-hexane, and the reaction bottle is sealed, vacuumed and connected to an oxygen balloon to make it at 1atm O 2 atmosphere, and reacted at room temperature for 26 hours; after the reaction, the reaction solution was detected by gas chromatography, and the conversion rate of the target product benzaldehyde was 83.8%, and the selectivity was 85.4%.
Embodiment 2
[0032] Example 2: Pd 3 Oxidation of Benzyl Alcohol Catalyzed by Cl@TNT in Ethanol Solution
[0033] Add 0.5mmol benzyl alcohol and 30mg catalyst Pd to a 10mL Schlenk reaction flask 3 Cl@TNT (Pd load is 1.5wt%) and 1mL ethanol, and the reaction bottle is sealed, vacuumed and connected to an oxygen balloon to make it at 1atm O 2 After the reaction, the reaction solution was detected by gas chromatography, and the conversion rate of the target product benzaldehyde was 95.7%, and the selectivity was 98.5%.
Embodiment 3
[0034] Example 3: Pd 3 Oxidation of Benzyl Alcohol Catalyzed by Cl@TNT in Toluene Solution
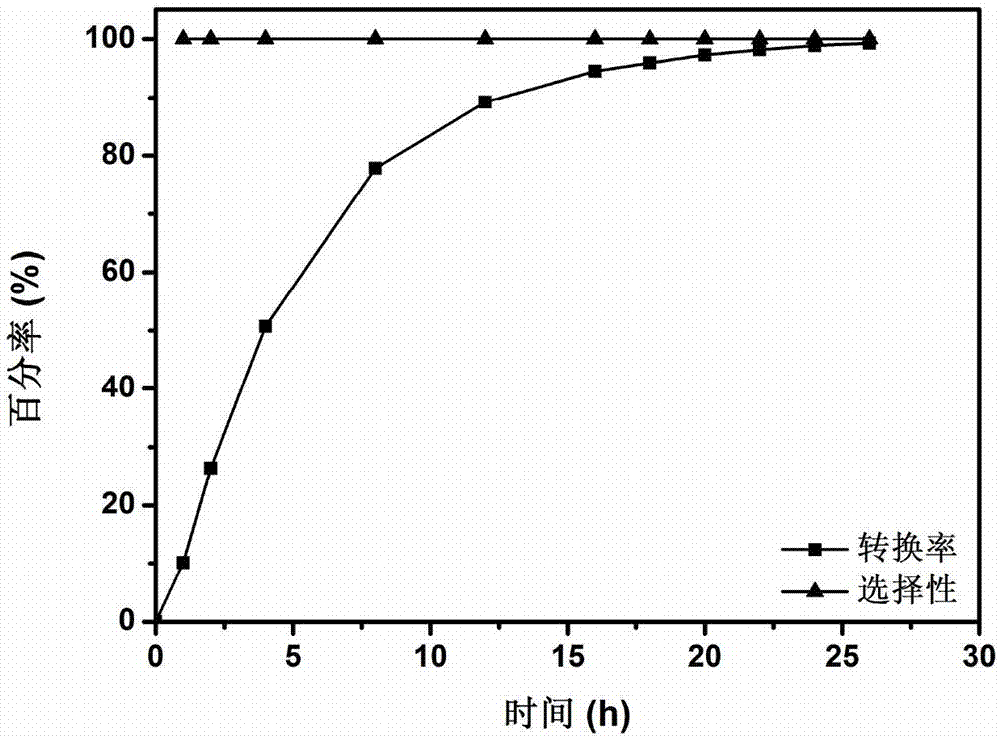
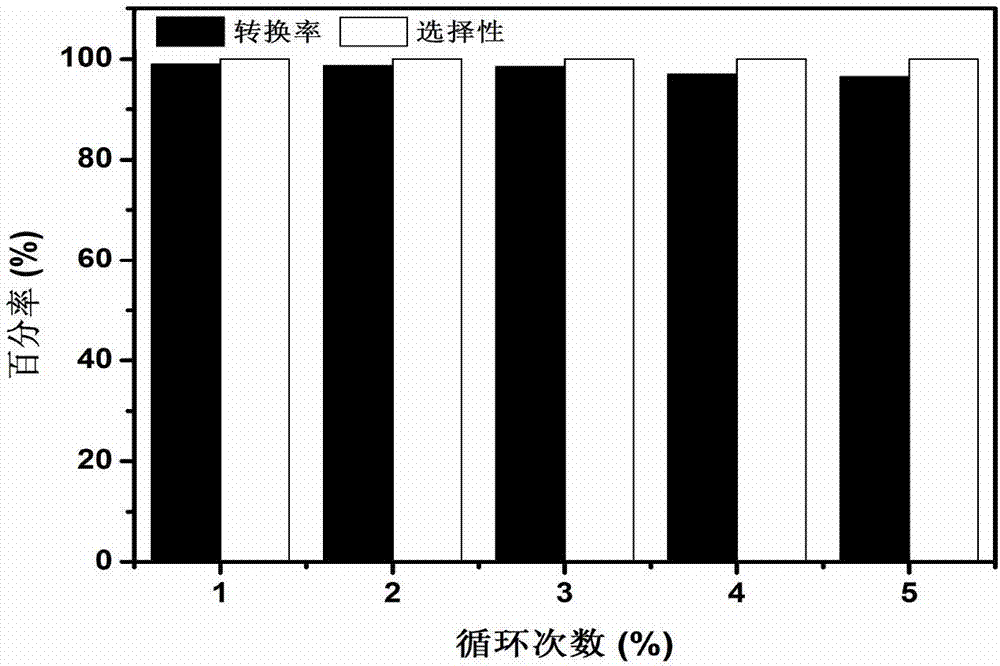
[0035] Into a 10mL Schlenk reaction flask, add 0.5mmol benzyl alcohol, catalyst 30mg Pd 3 Cl@TNT (Pd load is 1.5wt%) and 1mL ethanol, and the reaction bottle is sealed, vacuumed and connected to an oxygen balloon to make it at 1atm O 2 atmosphere, and reacted at room temperature for 26 hours; after the reaction, the reaction solution was detected by gas chromatography, and the conversion rate of the target product benzaldehyde was 99.3%, and the selectivity was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com