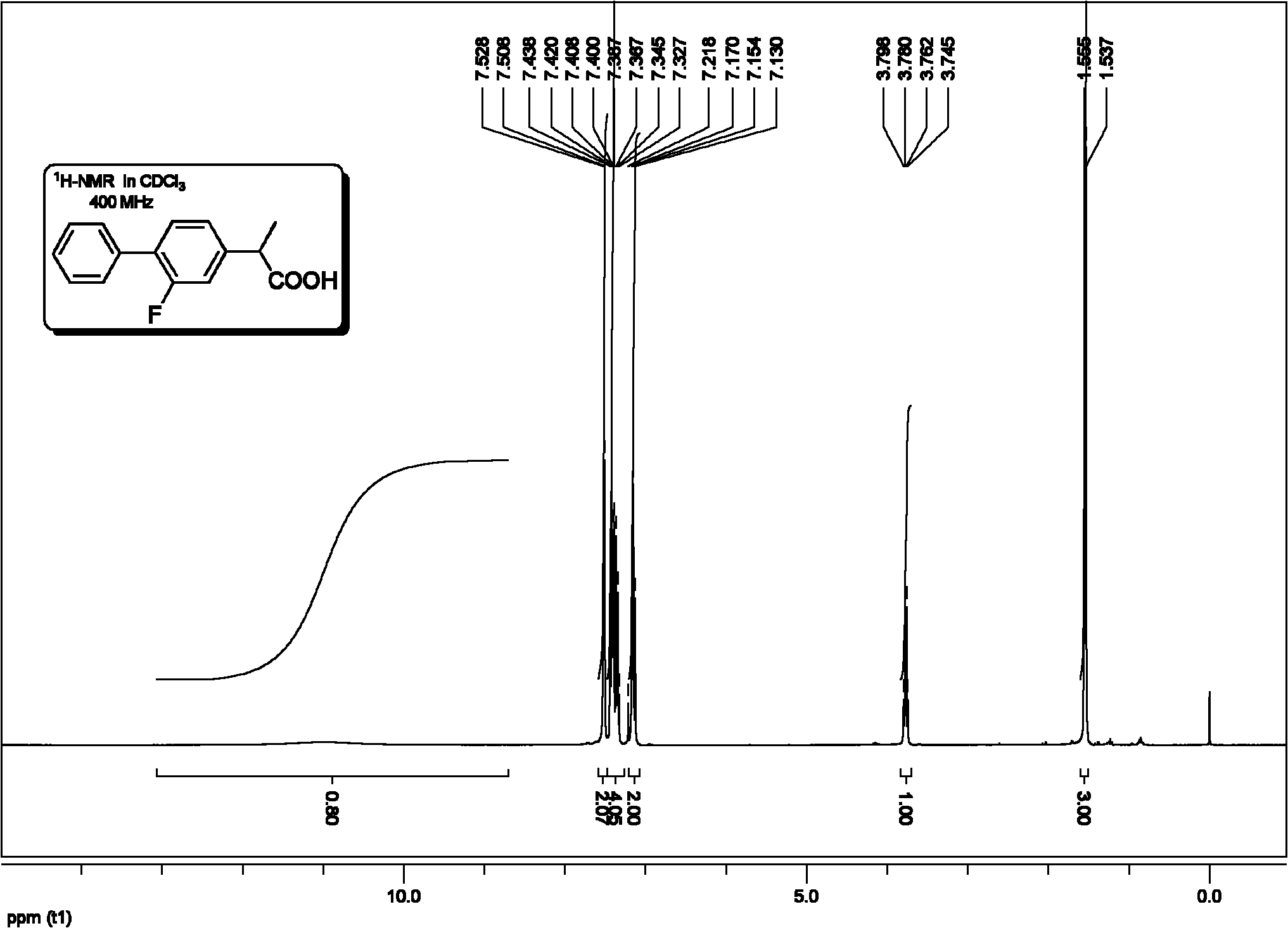
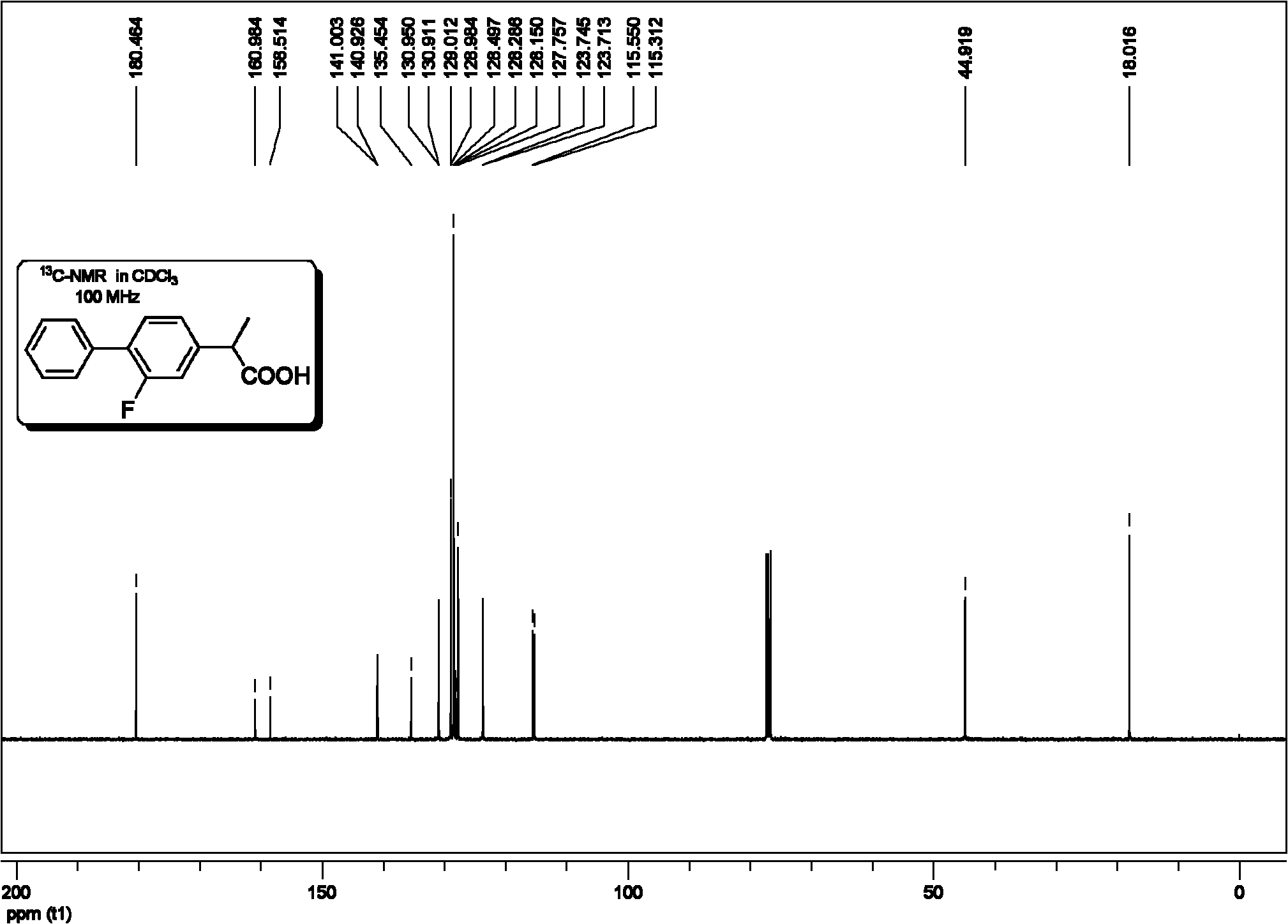
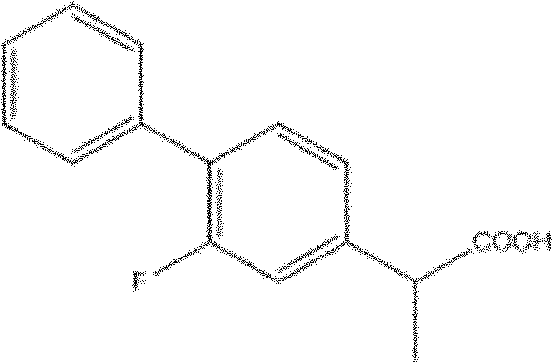
Method for synthesis of flurbiprofen
A technology of flurbiprofen and fluorobiphenyl, which is applied in the field of compound synthesis, can solve the problems of complex operation and long preparation route, and achieve the effect of wide source of raw materials, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Preparation of 2-(2-fluorobiphenyl-4-yl)propionitrile
[0078] In an oven-dried 100 mL Schlenk flask equipped with a magnet, add dipolyallylpalladium chloride (0.0146 g, 0.040 mmol), 9,9-dimethyl-4,5-bis(di tert-butylphosphino)xanthene (0.0693 g, 0.120 mmol), 4-bromo-2-fluorobiphenyl (10.04 g, 40 mmol) and potassium 2-cyanopropionate (6.58 g, 48 mmol). The lid is coated with a vacuum grease grinding plug, connected to the Schrank vacuum line, the air in the container is drained and filled with nitrogen, repeat 3 times, and 40mL of mesitylene is added under the reverse flow of nitrogen. After the addition, cover with a stopper, put it into a 140°C oil pan after clamping, and stir for 20 hours. After the reaction was completed, it was directly separated by chromatographic column to obtain white crystals with a yield of 95%.
[0079] (2) Preparation of 2-(2-fluorobiphenyl-4-yl)propionic acid
[0080] In a clean 100mL round bottom flask equipped with a magnet, add 2-...
Embodiment 2
[0083] (1) Preparation of 2-(2-fluorobiphenyl-4-yl)acetonitrile
[0084] Add palladium chloride (0.0071 g, 0.040 mmol), 2-bicyclohexylphosphine-2′, 6′-dimethoxybiphenyl (0.0493 g, 0.120mmol), 4-chloro-2-fluorobiphenyl (8.27g, 40mmol) and sodium cyanoacetate (5.14g, 48mmol). The lid is coated with a vacuum grease grinding plug, connected to the Schrank vacuum line, the air in the container is drained and filled with nitrogen, repeat 3 times, and 40mL of mesitylene is added under the reverse flow of nitrogen. After the addition, cover with a stopper, put it into a 140°C oil pan after clamping, and stir for 20 hours. After the reaction was completed, it was directly separated by chromatographic column to obtain white crystals with a yield of 96%.
[0085] (2) Preparation of 2-(2-fluorobiphenyl-4-yl)propionitrile
[0086] In a 150 ml round bottom flask equipped with a magnet was added 2-(2-fluorobiphenyl-4-yl)acetonitrile (7.60 g, 36 mmol), NaH (0.86 g, 36 mmol) and 80 mL of DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com