Thixotropic organic small molecule gel and preparation method thereof
A technology of small molecule gel and molecular gel factor, which is applied in the field of supramolecular chemistry to achieve the effect of simple process, low cost and wide application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 0.04g of small organic molecule gelling factor to 1.96g of JP-10, heat until the gelling factor is completely dissolved, let stand and cool to room temperature, and form a translucent gel. The gelling factor is a s-triazinetrione derivative with three alkylureidophenyl groups (n=16).
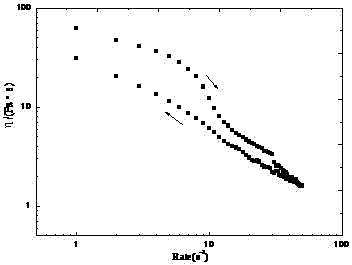
[0021] The rheological properties of the above-mentioned JP-10 gel were tested using a flat fixture with a distance of 1.0 mm and a shear rate ranging from 0.01 to 100 s -1 , whose rheological curve is as figure 1 shown.
[0022] It can be seen from the figure that the prepared JP-10 gel has good thixotropy. With the increase of shear rate, the viscosity of the gel system decreased rapidly; after the shear force was removed, the gel structure gradually recovered.
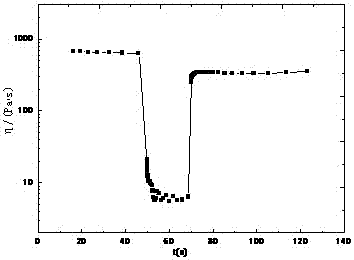
[0023] The JP-10 gel was sheared at high speed (500s -1 ) after breaking the ring, then shear at a low speed (0.1s -1 ) under recovery for a certain period of time, the results of its shear viscosity η with time t are ...
Embodiment 2
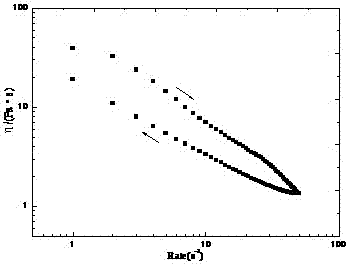
[0026] Add 0.06g of organic small molecule gelling factor to 1.94g of JP-10, heat until the gelling factor is completely dissolved, let it stand and cool to room temperature, and form a translucent gel, whose rheological curve is as follows image 3 shown. The gelling factor is a s-triazinetrione derivative with three alkylureidophenyl groups (n=18).
[0027] image 3 It shows that JP-10 gel has good thixotropy. After the gel is broken by high-speed shear, it recovers under low-speed shear, and its shear viscosity η changes with time t as follows: Figure 4 shown.
[0028] From Figure 4 It can be seen from the figure that after the JP-10 gel is destroyed, about 55% of the structure can be recovered within 50s. This destruction-recovery process can also be performed multiple times.
Embodiment 3
[0030] Add 0.17g of organic small molecule gelling factor to 1.83g of toluene, heat until the gelling factor is completely dissolved, and let stand to cool to room temperature to form a colorless transparent gel. The gelling factor is a s-triazinetrione derivative with three alkylureidophenyl groups (n=8).
[0031] After the gel is destroyed by external force, it forms a flowable liquid, and after standing still, it can form a gel again. This process can be repeated multiple times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com