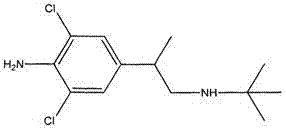
Magnetic particle chemiluminescence detection kit of clenbuterol and preparation method thereof
A chemiluminescence detection and chemiluminescence technology, applied in measurement devices, scientific instruments, instruments, etc., can solve the problems of expensive equipment, low sensitivity, and many influencing factors, and achieve good reagent stability, low background luminescence, and interference factors. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The present invention provides a clenbuterol magnetic particle chemiluminescence detection kit and a preparation method. In order to make the purpose, technical scheme and effect of the present invention clearer and clearer, the present invention will be further described in detail below.
[0023] The invention provides a clenbuterol magnetic particle chemiluminescence detection kit and a preparation method, wherein the magnetic particle chemiluminescence method detection kit for clenbuterol can adopt clenbuterol monoclonal Antibody-coupled magnetic particles, acridinium ester-labeled clenbuterol antigen, clenbuterol antigen-coupled magnetic particles, acridinium ester-labeled clenbuterol monoclonal antibody can also be used. The kit also includes clenbuterol calibrator, chemiluminescence pre-excitation solution A, chemiluminescence excitation solution B and cleaning solution for the above-mentioned acridinium ester action.
[0024] Specifically, the inner core of the m...
Embodiment 1
[0032] Embodiment 1: The set-up and preparation method of the magnetic particle chemiluminescence kit 1 for detecting clenbuterol includes the following steps:
[0033] 1. The composition of kit 1:
[0034] Construct a magnetic particle chemiluminescence kit for the detection of clenbuterol so that it contains the following components:
[0035] Clenbuterol monoclonal antibody coupled to carboxyl magnetic particles;
[0036] Acridinium ester-labeled clenbuterol antigen;
[0037] Clenbuterol series standard solution, the concentrations are: 0 μg / L, 0.01 μg / L, 0.06 μg / L, 0.36 μg / L, 2.16 μg / L, 21.6 μg / L, the buffer contains 1- 3% BSA and 0.1-0.3% PC300 in Tris-HCl solution;
[0038] Chemiluminescence pre-excitation solution A and chemiluminescence excitation solution B;
[0039] The cleaning solution is specifically Tris-HCl buffer solution (pH 7.2) with a concentration of 25 mmol / L, which contains NaCl and 0.05% Tween-20 with a concentration of 0.15 mol / L.
[0040] 2. Prepar...
Embodiment 2
[0058] Embodiment 2: The establishment and preparation method of a magnetic particle chemiluminescence kit 2 for detecting clenbuterol, comprising the following steps:
[0059] 1. The formation of kit 2:
[0060] Construct a magnetic particle chemiluminescence kit for the detection of clenbuterol so that it contains the following components:
[0061] Clenbuterol antigen coupled to carboxyl magnetic particles;
[0062] Acridinium ester-labeled clenbuterol monoclonal antibody;
[0063] Clenbuterol series standard solution, the concentrations are: 0 μg / L, 0.01 μg / L, 0.06 μg / L, 0.36 μg / L, 2.16 μg / L, 21.6 μg / L, the buffer contains 1- 3% BSA and 0.1-0.3% PC300 in Tris-HCl solution;
[0064] Chemiluminescence pre-excitation solution A and chemiluminescence excitation solution B;
[0065] The cleaning solution is specifically Tris-HCl buffer solution (pH 7.2) with a concentration of 25 mmol / L, which contains NaCl and 0.05% Tween-20 with a concentration of 0.15 mol / L.
[0066] 2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com