Recombination H1N1 subtype swine flu bivalent inactivated vaccine and preparation method and application thereof
A bivalent inactivated vaccine, H1N1 technology, applied in the field of recombinant H1N1 subtype swine influenza bivalent inactivated vaccine and its preparation, can solve the problems of difficult handling of chicken embryos, high consumption, antigenic variation, etc., and achieve benefits to the industry The effect of globalization and market application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of European Avian H1N1 Subtype SIV Recombinant Strain SH1 / PR8 and Classical H1N1 Subtype SIV Recombinant Strain G11 / PR8
[0030] Using European poultry H1N1 subtype swine influenza virus domestic epidemic strain A / swine / Shanghai / 1 / 2014 (H1N1) (SH1) as material, using RT-PCR to amplify HA and NA genes (SEQ ID NO.3 and SEQ ID NO.3 and NO.4), and clone it into a PBD vector to construct recombinant plasmids of HA and NA genes. The HA and NA gene recombinant plasmids and 6 internal gene (PB2, PB1, PA, NP, M and NS) recombinant plasmids derived from influenza virus strain A / Puerto Rico / 8 / 1934 (H1N1) (PR8) were co-transformed Infected 293T cells, and successfully constructed the recombinant European avian H1N1 subtype swine influenza vaccine strain SH1 / PR8. The recombinant vaccine strain SH1 / PR8 has higher virus titer and hemagglutination titer on MDCK cells or chicken embryos.
[0031] Using the classical H1N1 subtype swine influenza virus domestic ep...
Embodiment 2
[0032] Example 2 Preparation of Recombinant H1N1 Subtype Swine Influenza Bivalent Inactivated Vaccine
[0033] The recombinant European avian H1N1 subtype swine influenza virus (SH / PR8) and the classical H1N1 subtype swine influenza virus (G11 / PR8) were proliferated in large quantities on MDCK cells. The proliferated virus was inactivated by 0.1% formaldehyde solution at 37°C for 48 hours, and the HA hemagglutination titer was detected after the blind passage of the inactivated virus for 3 generations (if the HA hemagglutination titer cannot be detected, it means that the inactivated virus is safe, can enter the next emulsification operation). Recombinant inactivated viruses SH / PR8 and G11 / PR8 were passed through oil-emulsion adjuvant (MONTANIDE TM ISA 61VG, Sepic Company, France) was emulsified, and the inactivated virus was emulsified with the weight ratio of adjuvant to antigen at 60:40 according to the requirements of the adjuvant instructions, and prepared into an inact...
Embodiment 3
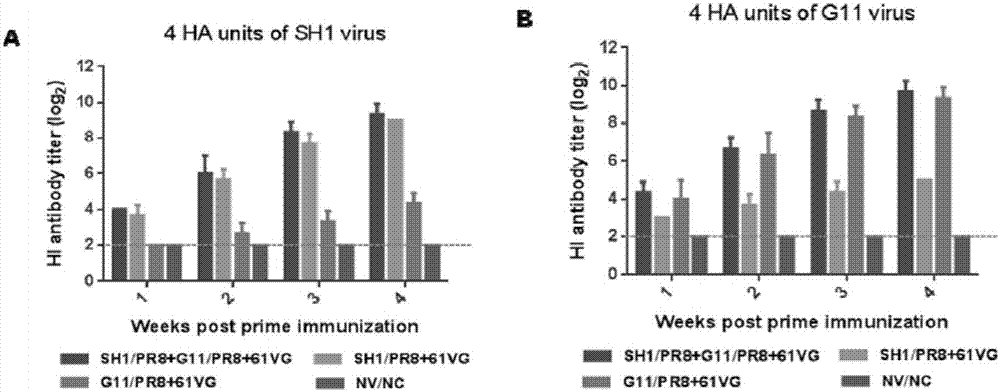
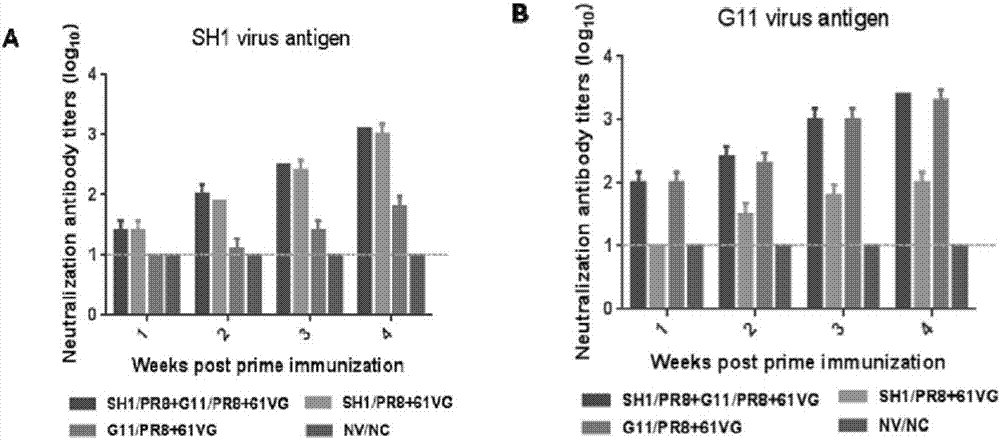
[0034] Example 3 Evaluation of Immunoprotective Efficacy of Recombinant H1N1 Subtype Swine Influenza Bivalent Inactivated Vaccine
[0035] 1. Procedures for evaluation of bivalent inactivated vaccine immunization and challenge using mice as a model
[0036] (1) Choose healthy SPF female BALB / c mice at the age of 6-8 weeks, and divide them into 5 groups at random, with 11 each in Group 1, Group 2, Group 3, and Group 4, and 22 mice in Group 5 (11 of which are used for attacking). Toxicity test, 11 were only used as environmental controls). Raise and manage the mice according to the requirements of the SPF level, and raise the mice normally for 3 days to familiarize the mice with the feeding environment, and collect blood from the orbital vein to collect the mouse serum as a blank control;
[0037] (2) The specific immunization procedures of the mice in the experimental groups (Group 1, Group 2, Group 3, Group 4, and Group 5) are shown in Table 1 below. Immunize 200 μl of inact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com