Method for preparation of BP (black phosphorene) modified electrode and detection of rutin
A technology for modifying electrodes and black phosphorene, applied in the direction of electrochemical variables of materials, etc., can solve the problems of poor stability, limited practical application value, easy decomposition, etc., and achieve high stability, good catalytic performance, and good electrochemical performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Electrochemical performance of the modified electrode
[0029] with K 3 [Fe(CN) 6 ] is an electrochemical probe, and the electrochemical performance of different electrodes is studied by cyclic voltammetry. The cyclic voltammetry curve is as follows figure 1 shown. A pair of redox peaks with good peak shape appeared on the bare GCE electrode (curve a), the redox peak currents were 15.87 μA (Ipa) and 16.10 μA (Ipc), respectively, and the peak potential difference was 90.0 mV; while in BP-PEDOT: The peak current drop on PSS / GCE is significantly enhanced (curve b), Ipa is 45.65 μA, and Ipc is 45.45 μA, which are 2.88 times and 2.82 times the peak current of the bare electrode, respectively. This is due to the accelerated [Fe(CN) 6 ] 3- / 4- the electron transfer rate.
Embodiment 2
[0030] Example 2 Solving the effective area of the modified electrode
[0031] Using the Randles-Sevcik formula I pa (A)=(2.69×10 5 ) n 3 / 2 AD 1 / 2 C 0 υ 1 / 2 , where n is the electron transfer number; A is the effective area (cm 2 ); D is the diffusion coefficient of potassium ferricyanide solution (7.6×10 -6 cm s -1 ), C 0 is the concentration of potassium ferricyanide (1mmol / L), and the effective area of BP-PEDOT:PSS / GCE is calculated to be 0.196 cm 2 , whose value is 8.34 times the effective area of GCE (0.0235 cm 2 ), indicating that the presence of PEDOT:PSS can effectively increase the effective area of the electrode, provide more active sites on the electrode surface, and improve the electrode performance.
Embodiment 3
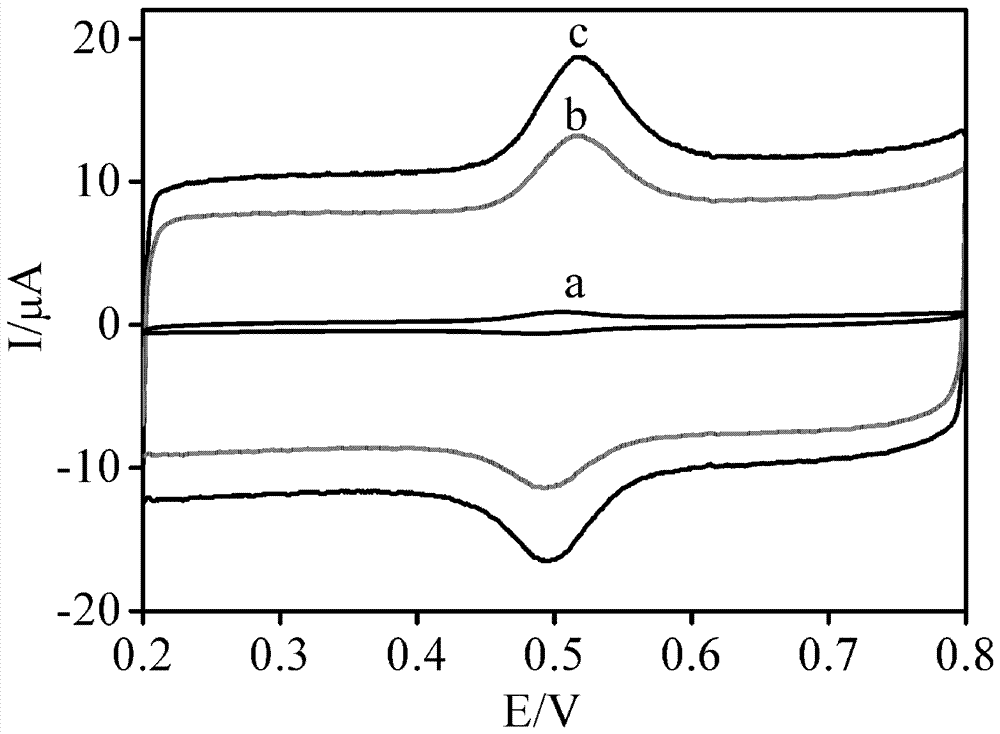
[0032] Example 3 Research on the electrochemical behavior of rutin
[0033] In 0.1 mol / L PBS buffer solution with pH 2.5, 1.0×10 -5 The electrochemical behavior of mol / L rutin on different modified electrodes, the results are as follows figure 2 shown. On the bare GCE (curve a), rutin has a pair of redox peaks at 0.505V and 0.485V, ΔEp is 20 mV, Ipa and Ipc are 0.558 μA and 0.324 μA, respectively, indicating that rutin can realize its direct oxidation on the surface of GCE. electrochemistry. Curve b is the electrochemical behavior curve of rutin on PEDOT:PSS / GCE, Epa and Epc are located at 0.516 V and 0.493 V, ΔEp is 23 mV, Ipa and Ipc are 4.96 μA and 3.12 μA, and their values are GCE 8.89 times and 9.63 times of , indicating that the presence of PEDOT:PSS accelerates the electron transfer rate of rutin on the electrode surface and improves the electrochemical response. Curve c is the electrochemical behavior curve of rutin on BP-PEDOT:PSS / GCE. 13.69 times and 16.48 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com