Composition containing amino amantadine mononitrate compounds and used for eyes as well as preparation and application of composition
An ophthalmic composition and amantadine technology, applied in the field of medicine, can solve the problems of increasing the possibility of drug toxicity, difficult to achieve effective concentration, poor clinical compliance, etc., and achieve long release duration, lasting effect, and use compliance good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
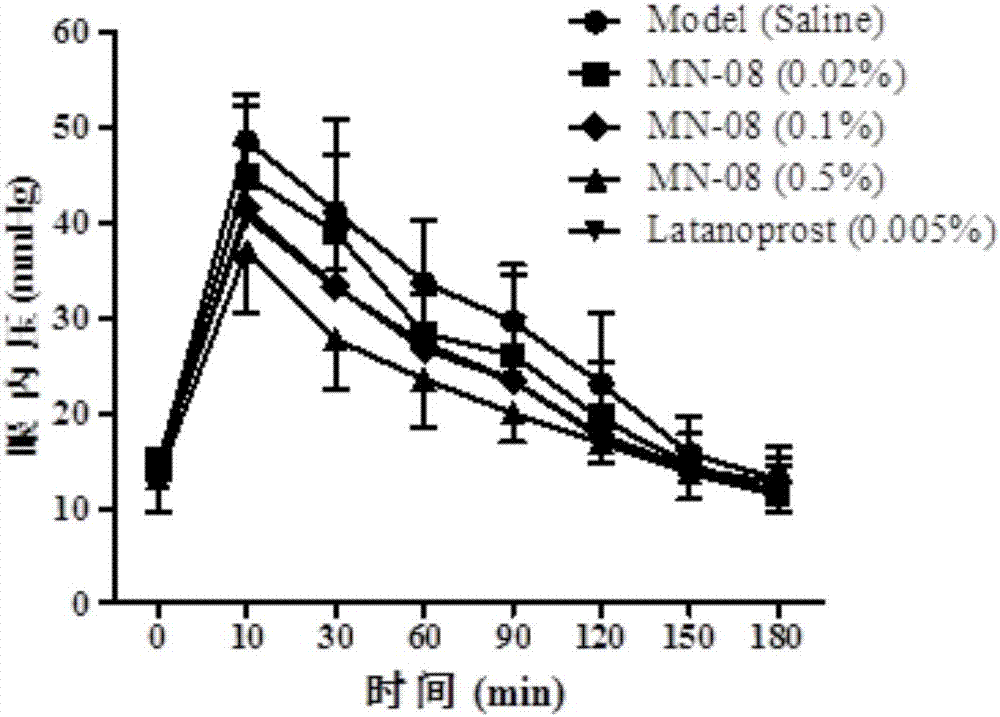
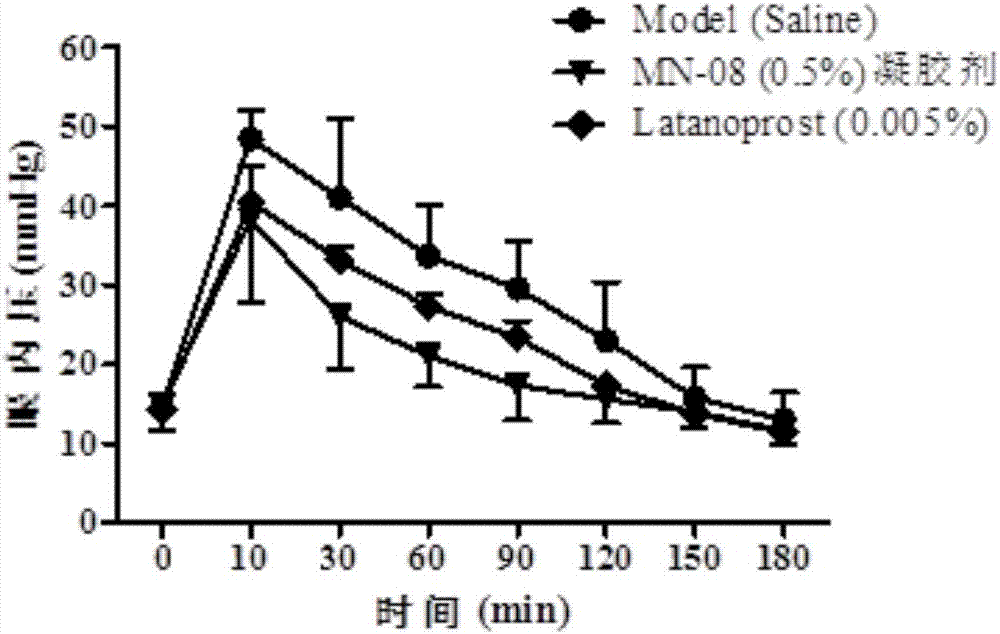
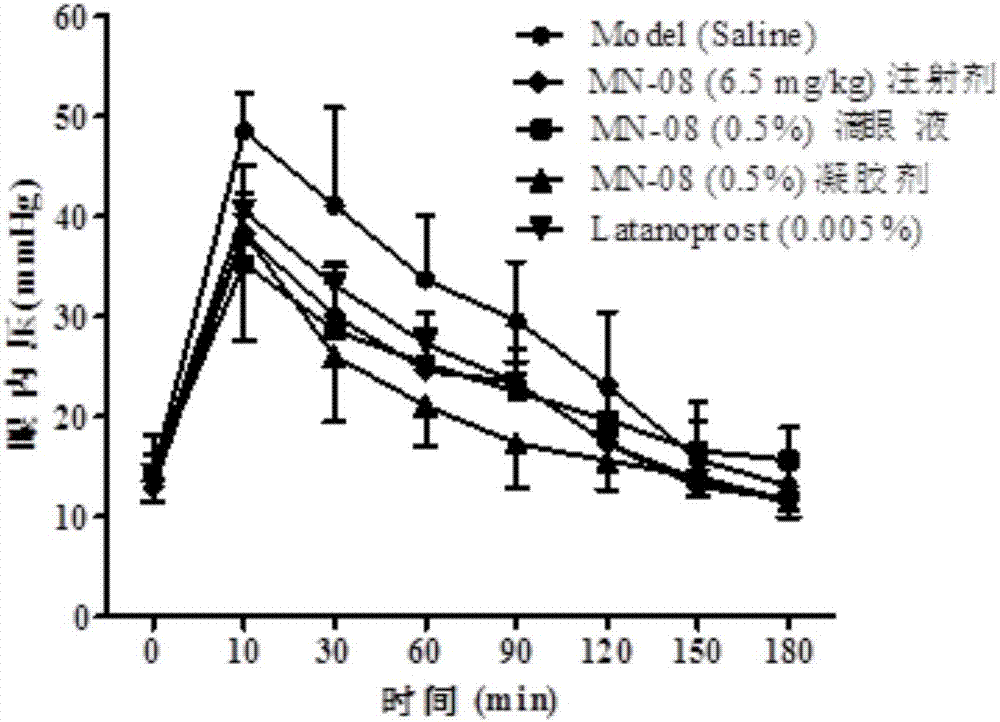
[0035] 1. Production of New Zealand Rabbit Transient Glaucoma Model
[0036] New Zealand rabbits with a body weight ranging from 1.5 to 2 kg were selected as model animals to make a high intraocular pressure model. The New Zealand rabbits were kept in a clean animal room for 3 days, and the experiment started after reaching the required body weight range. First, use pentobarbital sodium (3%, 1ml / kg) to anesthetize until there is no blink reflex; then, use compound tropicamide eye drops to dilate the pupils, and oxybucaine hydrochloride eye drops for local anesthesia. Boom eye drops to prevent dry eyes, levofloxacin eye drops to reduce inflammation, and iodine to disinfect around the eyes; inject 0.1ml of 5% hypertonic saline into the vitreous, using a 33G needle, and the injection site is the limbus After 3.5-4mm, the injection depth is 5-7mm; after withdrawing the needle. Tonolab intraocular pressure gauge was used to measure the change of intraocular pressure 1 min after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com