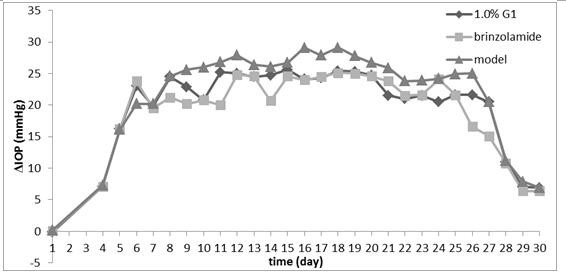
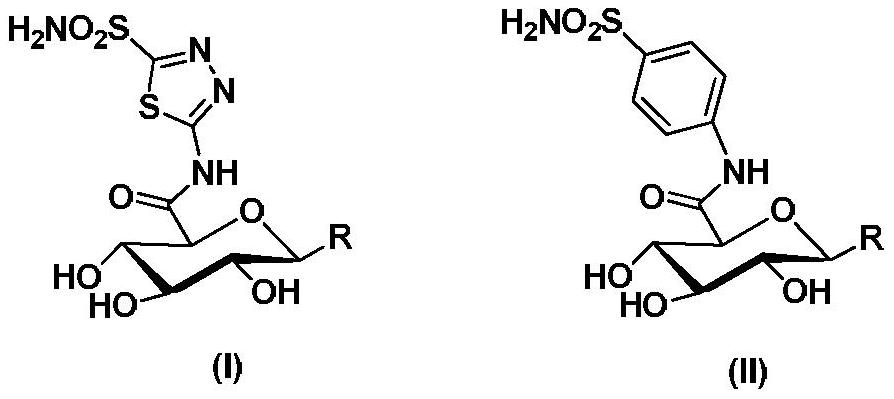
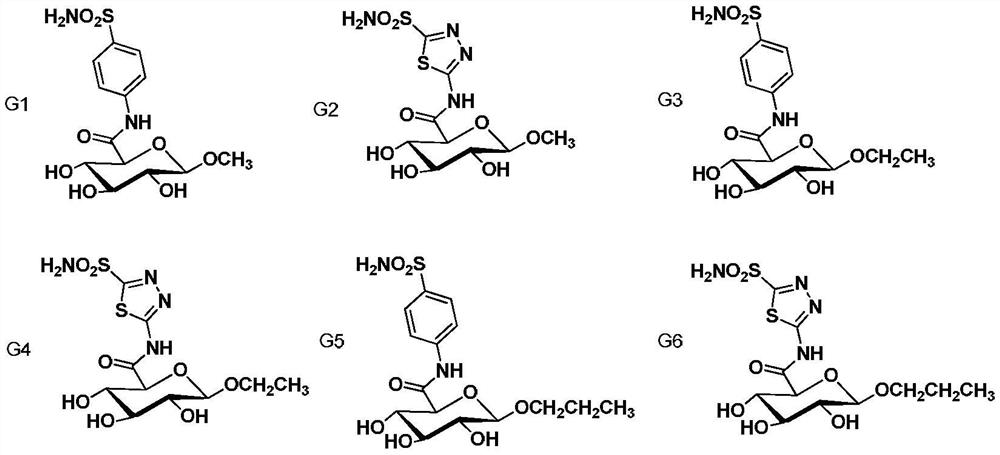
Preparation method and medical use of sulfonamide compounds with glucuronic acid structure
A technology of glucuronic acid and compounds, which is applied in the field of preparation and medical application of sulfonamide compounds with glucuronic acid structure, can solve problems such as eye irritation, unsatisfactory binding ability of carbonic anhydrase, toxic and side effects, etc. The effect of internal pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: N-(1'-O-methyl-β-D-glucuronyl)-4-aminophenylsulfonamide (G1)
[0025] At room temperature, dissolve 15.0 g of glucuronolactone 1 in 70 mL of methanol, add 2.4 mL of 1 mol / L sodium methoxide / methanol solution, and react in the dark until all solids disappear, add 0.14 mL of glacial acetic acid to adjust the pH to 7, and reduce Press and concentrate to obtain brown viscous syrup 2.
[0026] Dissolve the above product in 70mL of pyridine, add 70mL of benzoyl chloride dropwise in an ice-water bath, and react for 5 hours. After the solution is clarified, add 200mL of water, continue to stir for 20 minutes, and extract 3 times with dichloromethane. Washed with water, dilute hydrochloric acid and saturated sodium bicarbonate solution, anhydrous Na 2 SO 4 dry. After filtration, dichloromethane was distilled off under reduced pressure to obtain intermediate 3.
[0027] Dissolve the above product in 70mL of dichloromethane, add 70mL of hydrogen bromide acetic acid ...
Embodiment 2
[0031] Example 2: N-(1'-O-methyl-β-D-glucuronyl)-4-amino-1,3,4-thiadiazol-2-yl-sulfonamide (G2)
[0032] The preparation method of the compound in Example 2 is the same as in Example 1, except that 5-amino-1,3,4-thiadiazol-2-yl-sulfonamide is used instead of p-aminobenzenesulfonamide. m.p 200.9–202.6°C; 1 H NMR (600MHz, DMSO-d 6 ) δ8.36(s,2H),5.27(d,J=5.1Hz,1H),5.24(d,J=5.0Hz,1H),4.19(d,J=7.8Hz, 1H),4.12(s, 1H), 4.00(d, J=9.7Hz, 1H), 3.49(t, J=9.3Hz, 1H), 3.40(s, 3H), 3.23 (td, J=9.0, 4.7Hz, 1H), 3.07( td,J=8.5,5.0Hz,1H). 13 C NMR (151MHz, DMSO-d 6 )δ168.88,165.17,161.48,105.08,76.48,76.06,73.37,71.59,56.98,49.12. ESI-MS(m / z):368.8[M-H] – ,393.2[M+Na] + ;HRMS(ESI):Calcd.for[M-H] – C 9 h 13 N 4 o 8 S 2 :369.0253,Found 369.0275[M-H] – .
Embodiment 3
[0033] Example 3: N-(1'-O-ethyl-β-D-glucuronyl)-4-aminophenylsulfonamide (G3)
[0034] The preparation method of the compound of Example 3 is the same as that of Example 1, except that ethanol is used instead of methanol. m.p 231.1–234.2°C; 1 H NMR (600MHz, DMSO-d 6 )δ10.46(s,1H),7.82(s,2H),7.77(d, J=8.9Hz,2H),7.27(s,2H),5.39(s,1H),5.18(s,2H), 4.24(d, J=7.8Hz, 1H), 3.79 (ddd, J=9.8, 8.2, 5.5Hz, 2H), 3.54–3.45(m, 2H), 3.21(t, J=9.0Hz, 1H), 3.06 (t,J=8.4Hz,1H),1.13(d,J=4.5Hz,3H). 13 C NMR (151MHz, DMSO-d 6 )δ168.10, 142.20, 139.11, 127.15, 119.47, 103.71, 77.36, 76.82, 73.56, 71.58, 64.84, 15.66. ESI-MS(m / z): 374.9[M-H] – ,399.2[M+Na] + ;HRMS(ESI):Calcd.for[M-H] – C 14 h 19 N 2 o 8 S:375.0840,Found 375.0884[M-H] – .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com