Composition granule used for cough, excessive phlegm and asthma and preparation method of composition granule
A composition and granule technology, applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of low bioavailability, inconvenient to take and carry, poor taste, etc., and achieve no toxic side effects, Delicious taste and the effect of overcoming the inconvenience of decoction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
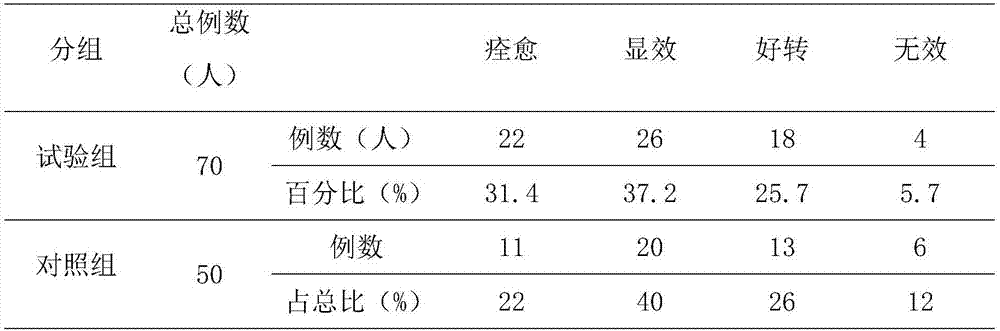
Examples
preparation example Construction
[0033] A preparation method for composition granules for cough, phlegm, and asthma, comprising the following steps:
[0034] S1: Weigh raw materials according to the following parts by weight: 90-110 parts of orange red, 70-90 parts of almonds, 40-60 parts of mulberry leaves, 40-60 parts of bellflower, 40-60 parts of orange peel, 20-40 parts of poria cocos, perilla 20-40 parts of seeds, 10-30 parts of licorice, 50-60 parts of excipients, 15-25 parts of binders, 10-12 parts of lubricants, and 5-8 parts of preservatives;
[0035] The excipient is at least one of powdered sugar, lactose, and cyclodextrin;
[0036] The binder is at least one of starch slurry, sodium carboxymethylcellulose, and carboxypropylmethylcellulose;
[0037] Described lubricant is magnesium stearate or talc;
[0038] The preservative is at least one of benzoic acid, sorbic acid, and potassium sorbate;
[0039] S2: Take the raw materials weighed in step S1 and put them into an extraction tank, add 10-15 tim...
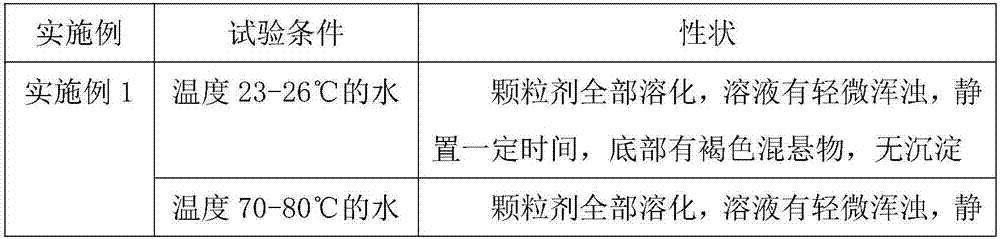
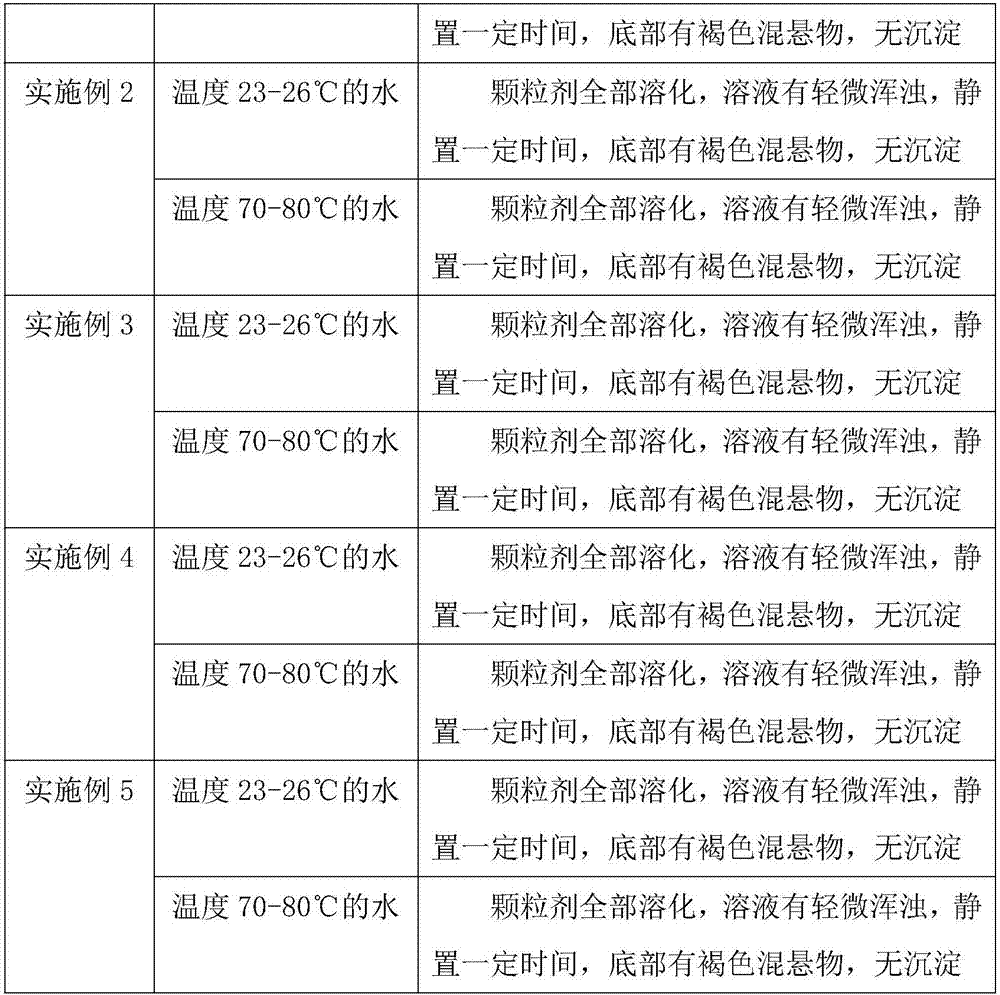
Embodiment 1
[0045] A preparation method for composition granules for cough, phlegm, and asthma, comprising the following steps:
[0046] S1: Weigh raw materials according to the following parts by weight: 90 parts of orange red, 70 parts of almond, 40 parts of mulberry leaf, 40 parts of bellflower, 40 parts of orange peel, 20 parts of poria cocos, 20 parts of perilla seed, 10 parts of licorice, 50 parts of excipient 15 parts of adhesive, 10 parts of lubricant, 5 parts of preservative;
[0047] The excipient is powdered sugar;
[0048] The binder is starch slurry;
[0049] Described lubricant is magnesium stearate;
[0050] The preservative is benzoic acid;
[0051] S2: Take the raw materials weighed in step S1 and put them into an extraction tank, add 10 times the amount of water to decoct for 1.5 hours, filter, add 8 times the amount of water to decoct the medicinal residues for 1 hour, filter, combine the filtrates, and concentrate the filtrates to relative density Thick paste of 1....
Embodiment 2
[0057] A preparation method for composition granules for cough, phlegm, and asthma, comprising the following steps:
[0058] S1: Weigh raw materials according to the following parts by weight: 95 parts of orange red, 75 parts of almond, 45 parts of mulberry leaf, 45 parts of bellflower, 45 parts of orange peel, 25 parts of poria cocos, 25 parts of perilla seed, 15 parts of licorice, 52 parts of excipient 18 parts of adhesive, 11 parts of lubricant, 6 parts of preservative;
[0059] The excipient is lactose;
[0060] Described binding agent is sodium carboxymethyl cellulose;
[0061] Described lubricant is talcum powder;
[0062] The preservative is sorbic acid;
[0063] S2: Take the raw materials weighed in step S1 and put them into an extraction tank, add 11 times the amount of water to decoct for 2 hours, filter, add 9 times the amount of water to decoct the medicinal residues for 1.5 hours, filter, combine the filtrates, and concentrate the filtrates to relative density ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com