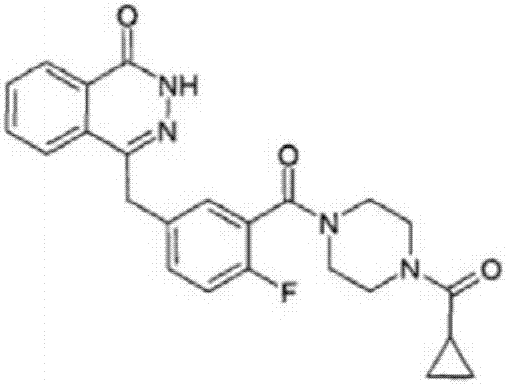
Olaparib composition capsule
A composition and capsule technology, applied in the field of olaparib composition capsules, can solve the problems of high water content, large amount of carrier, and no further prompts are given.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of olaparib capsules:
[0033] Separately pulverize the drug ingredients olaparib, starch, di-tert-butyl-p-cresol, sodium alginate, glyceryl behenate, pass through a 80-mesh sieve, mix them evenly and send them to a dry granulator for granulation, 18-mesh granulation , Capsule Filling.
[0034] experiment method:
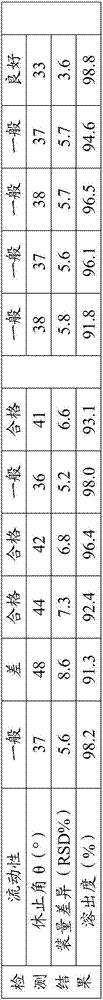
[0035] 1. Liquidity test:
[0036] The fluidity of a solid cannot be expressed by a single characteristic value, and it is often expressed by the angle of repose. It usually refers to the largest angle formed by the free slope of the powder accumulation layer and the horizontal plane. The smaller the angle of repose, the smaller the friction, and the better the fluidity. It is generally believed that when θ≤30 degrees, the fluidity is good, and when θ≤40 degrees, it can meet the fluidity requirements in the production process. The fluidity of powder has a great influence on the weight difference and normal operation of granules, capsu...
experiment example 1
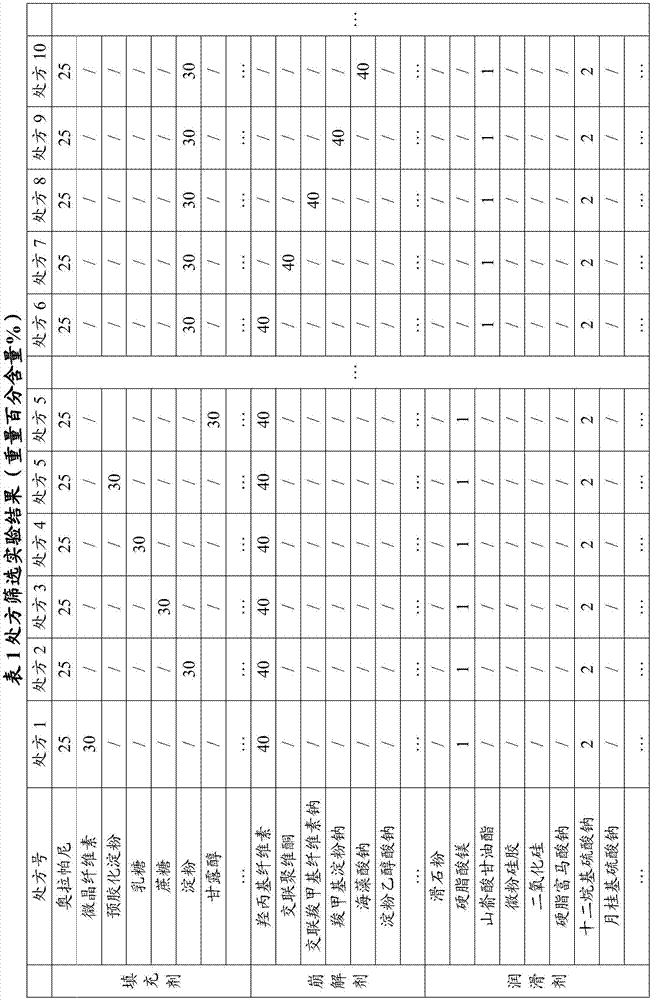
[0042] Experimental Example 1: Prescription Screening Experiment
[0043]
[0044]
[0045] Since olaparib is easy to absorb moisture and is unstable to heat and humidity, the dry granulation method is preferred to prevent the influence of the temperature of the moisture agent on the raw and auxiliary materials. Put it into a dry granulator for granulation, 18-mesh granulation, and capsule filling.
[0046] There are too many screening experimental data, and here are only some important experimental data. After a large number of experimental screening, the inventor found that when hydroxypropyl cellulose is combined with starch or lactose, the dissolution rate is not good, especially when hydroxypropyl cellulose is combined with starch or lactose. When the starch is combined and applied, the fluidity is very poor, and the difference in loading is obvious; when the lubricant is changed to glyceryl behenate, the fluidity is significantly improved, but the dissolution rate ...
experiment example 2
[0047] Experimental Example 2: Screening experiment of dosage of glyceryl behenate and sodium alginate
[0048] This experimental example is used as a dosage screening experiment of glyceryl behenate and sodium alginate when preparing olaparib capsules, and the weight percentage of each raw material is controlled: olaparib 30%-35%, starch 50.4% -53.5%, di-tert-butyl-p-cresol 0.1%-0.3%, on this basis, adjust the weight percent content of glyceryl behenate and sodium alginate.
[0049] The amount of disintegrating agent sodium alginate has a great influence on the disintegration time and dissolution rate, and if the amount is too small, it cannot meet the requirements of disintegration and dissolution. Sodium alginate aqueous solution is a viscous disintegrating agent, the larger the dosage, the slower the disintegration and dissolution rate. Therefore, the inventors selected 14%-18% by weight after a large number of experimental screenings.
[0050] Table 2 The amount screeni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com