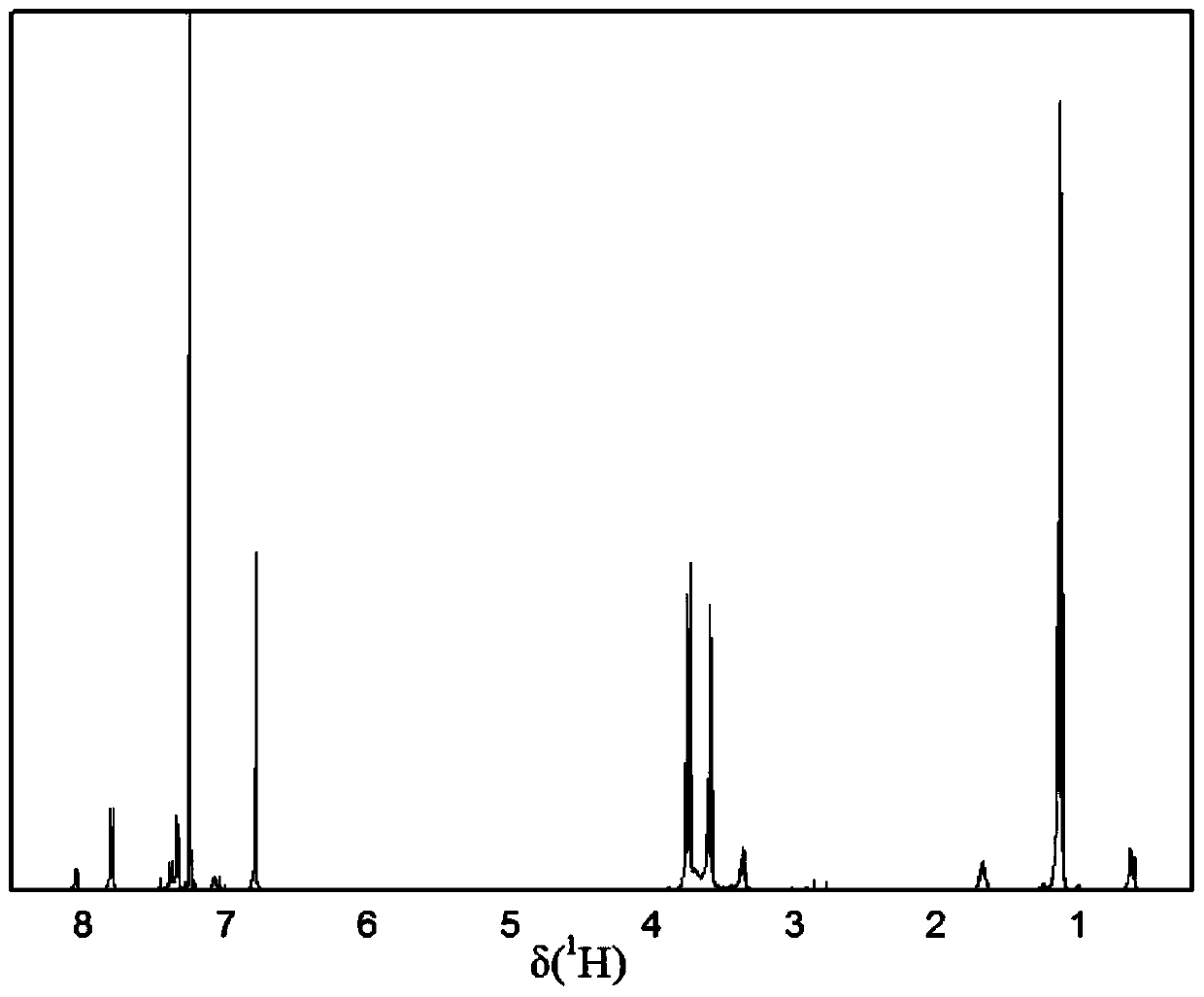
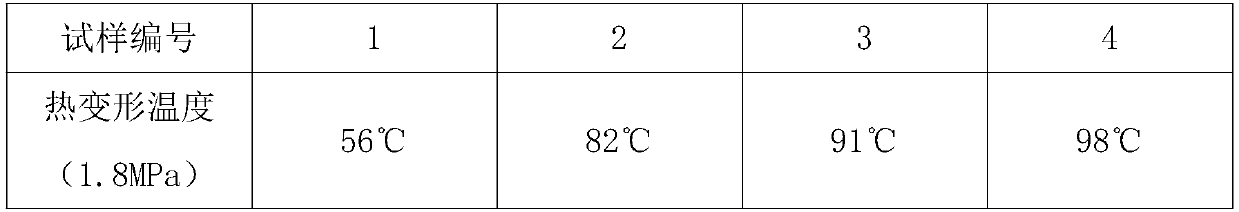
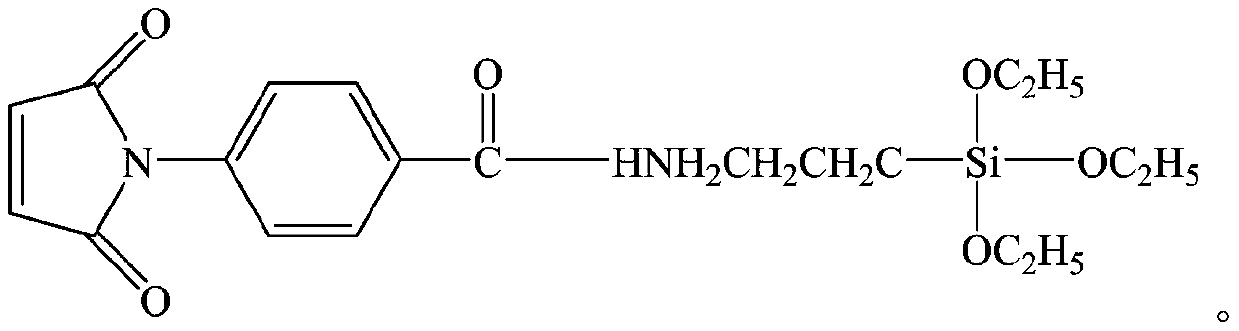A kind of copolymer composition of n-phenylmaleimide derivatives
A technology of maleimide and derivatives, applied in the field of copolymer compositions of N-phenylmaleimide derivatives, can solve problems such as limited heat resistance temperature of polymers, and achieve good effect and heat resistance. The effect of improving performance and increasing the field of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis steps of CPMA: (1) Dissolve 1.078g MAH in 10mL of DMF; (2) Dissolve 1.51g of p-aminobenzoic acid in 10mL of DMF, and then add the solution dropwise to the DMF solution of MAH within 15-30min medium; (3) react at 40 degrees for 6 hours; (4) after the reaction, cool the reaction system to room temperature, and then extract it with crushed ice; (5) Dry the CPMA in an oven at 40°C.
[0019] Weigh 2.17g of N-(4-carboxyphenyl)maleamic acid (CPMA) synthesized in the first step, and dissolve it in DMF. Add 0.2g of catalyst anhydrous sodium acetate, 2mL of dehydrating agent acetic anhydride, 0.2g of polymerization inhibitor hydroquinone, raise the temperature, control the temperature between 50-55°C, react for 2h, the solution becomes light yellow and transparent, After cooling to room temperature, let it stand still, pour the solution into crushed ice to precipitate a large number of yellow needle-like crystals, then carry out suction filtration, washing, and dry...
Embodiment 2
[0025] Synthesis steps of CPMA: (1) Dissolve 10.78g MAH in 10mL of DMF; (2) Dissolve 15.1g of p-aminobenzoic acid in 100mL of DMF, and then add the solution dropwise to the DMF solution of MAH within 15-30min medium; (3) react at 40 degrees for 6 hours; (4) after the reaction, cool the reaction system to room temperature, and then extract it with crushed ice; (5) Dry the CPMA in an oven at 40°C.
[0026] Weigh 21.7g of N-(4-carboxyphenyl)maleamic acid (CPMA) synthesized in the first step, and dissolve it in DMF. Add 2g of catalyst anhydrous sodium acetate, 20mL of dehydrating agent acetic anhydride, 2g of polymerization inhibitor hydroquinone, raise the temperature, control the temperature between 50-55°C, react for 2h, the solution becomes light yellow and transparent, cool to After standing at room temperature, the solution was poured into crushed ice to precipitate a large number of yellow needle-like crystals, and then filtered, washed and dried to obtain pure light yello...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat deflection temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com