Method for co-producing monopotassium phosphate and nitrogen phosphorus and potassium compound fertilizer
A technology of nitrogen, phosphorus and potassium compound fertilizer and potassium dihydrogen phosphate, which is applied in the direction of urea compound fertilizer, ammonium orthophosphate fertilizer, alkaline orthophosphate fertilizer, etc., can solve the problem of insufficient utilization of raw materials for additional product mining, production equipment, and production environment impact, large dosage of phosphoric acid, etc., to achieve the effect of improving the production environment, improving the grade and grade, and reducing equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
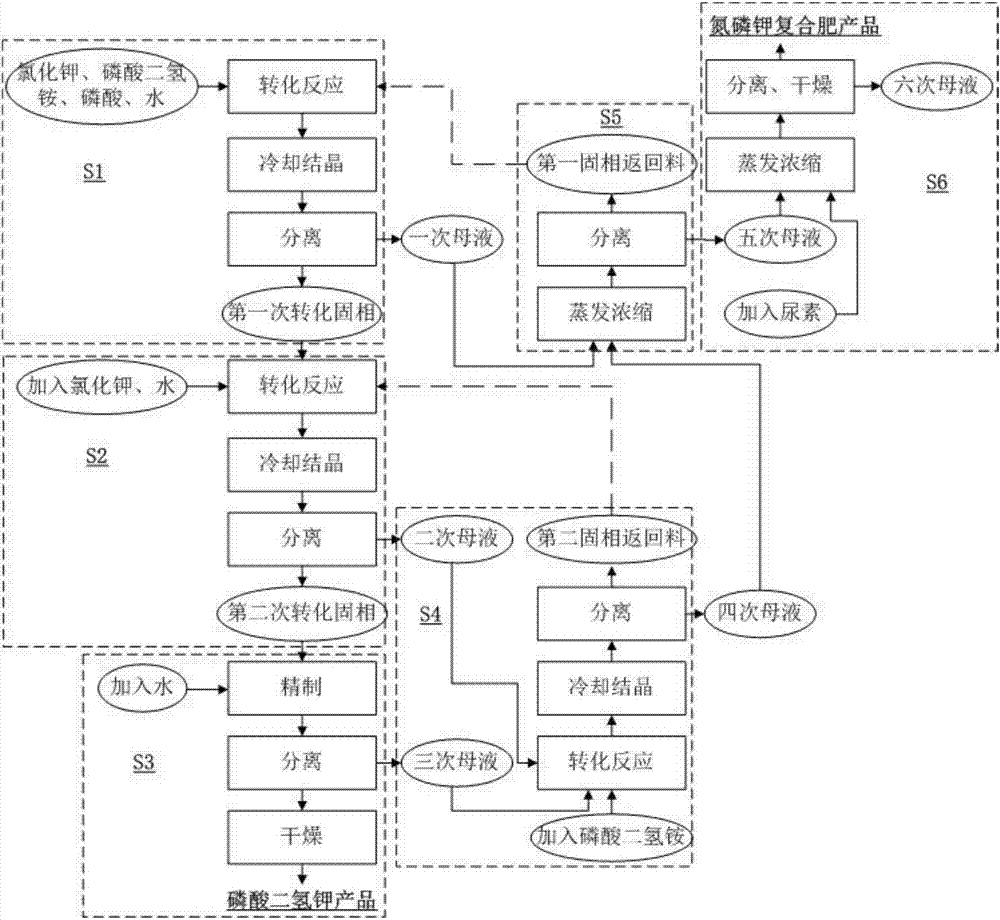
[0040] Such as figure 1 As shown, the process flow chart of the method for the coproduction of potassium dihydrogen phosphate and nitrogen, phosphorus and potassium compound fertilizers provided by the embodiment of the present invention, the method comprises the following steps:
[0041]S1. Add 400kg potassium chloride, 600kg ammonium dihydrogen phosphate and 1500kg-2000kg water into the reaction tank, add 40kg phosphoric acid to adjust the pH, the reaction temperature is 80°C-110°C, and the reaction time is 30min. Cool to 20° C. after the reaction, and separate and obtain 1000 kg of the first converted solid phase and 2000 kg of the first mother liquor. Wherein, the solid phase is converted to ammonium dihydrogen phosphate for the first time.
[0042] S2. The 1000kg of the first converted solid phase obtained in step S1 is converted and reacted with 500kg of potassium chloride and 3500kg to 4500kg of water in the reaction tank. The reaction temperature is 80°C to 110°C and ...
Embodiment 2
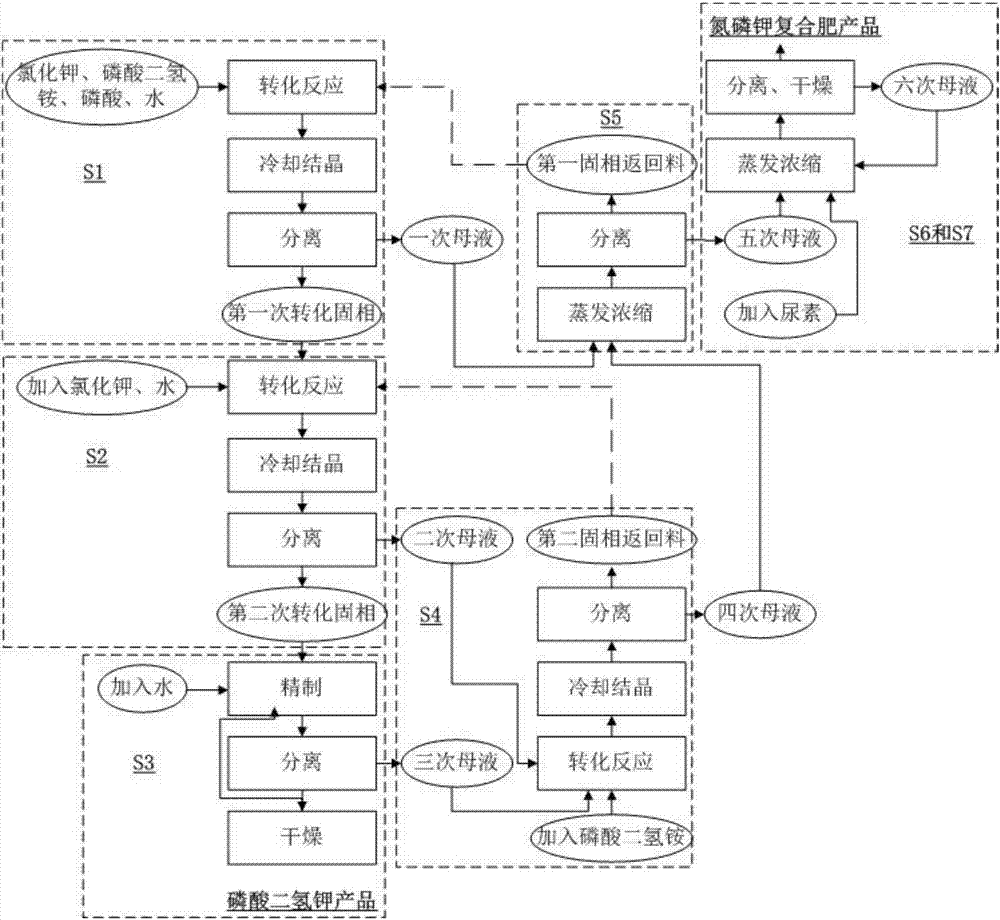
[0048] This embodiment provides a method for the joint production of potassium dihydrogen phosphate and nitrogen, phosphorus and potassium compound fertilizers, combining figure 2 As shown, the difference between the present embodiment and the first embodiment is that the method also includes the implementation of the S7 step after the implementation of the S6 step:
[0049] S7. Mix the five mother liquors obtained in step S5, the six mother liquors obtained in step S6, and urea at a mass ratio of 1:0.2-1:0.2-1, and repeat the process of step S6.
Embodiment 3
[0051] The present embodiment provides a method for co-production of potassium dihydrogen phosphate and NPK compound fertilizer, comprising the following steps:
[0052] S1. Add 400kg of potassium chloride, 700kg of ammonium dihydrogen phosphate and 1600kg to 2100kg of water into the reaction tank, add 40kg of phosphoric acid to adjust the pH, the reaction temperature is 80°C to 110°C, and the reaction time is 30min. After the reaction, it was cooled to 20° C., and 1100 kg of the first converted solid phase and 2100 kg of the first mother liquor were obtained by separation. Wherein, the solid phase is converted to ammonium dihydrogen phosphate for the first time.
[0053] S2. The 1100 kg of the first converted solid phase obtained in the step S1 is converted and reacted with 500 kg of potassium chloride and 4500 kg of water in the reaction tank. The reaction temperature is 80° C. to 110° C., and the reaction time is 30 minutes. After the reaction, it was cooled to 20° C., and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com