A kind of preparation method of 3-aminobutanamide compound
An aminobutyramide and compound technology, which is applied in the field of preparation of 3-aminobutanamide compounds, can solve the problems that liquid ammonia cannot be directly recovered and recycled, is not suitable for industrial production, requires high pressure and the like, and achieves easy operation and environmental protection. Friendly, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
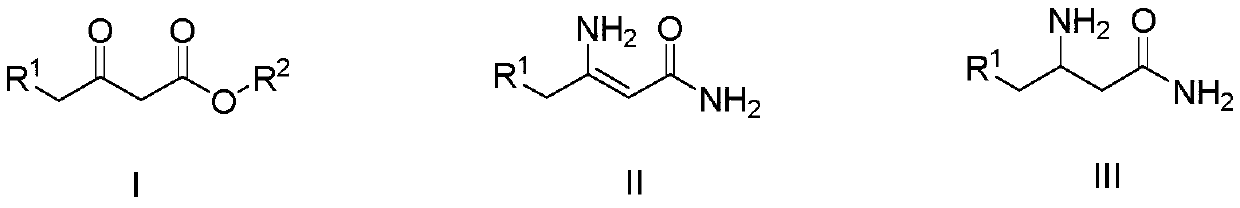
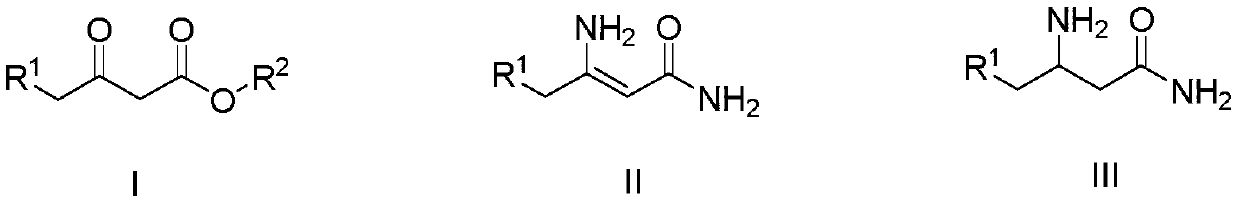
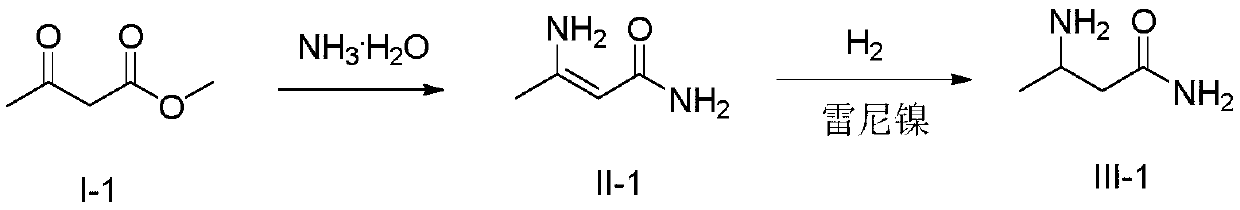
[0026] (1) Add 37.5% (mass fraction) ammoniacal liquor (91g, 2.00mol) in the 250mL four-necked round bottom flask that is equipped with mechanical stirring, 3-oxobutanoic acid methyl ester (11.6 mol) shown in formula (I-1) g, 0.10mol), react at 30°C, and after liquid chromatography detects that the reaction is complete, filter the reaction solution, and the filtrate can be directly applied to the next batch, and the resulting filter cake is soaked with water for 2 to 3 times, and placed in an oven at 50 After drying at ~60°C, 8.65 g of 3-aminobutenamide represented by formula (II-1) was obtained. The purity by liquid chromatography was 98.2%, and the yield was 84.8%. The detection condition of liquid chromatography is: C18 column, and the mobile phase volume ratio is: acetonitrile: water = 60:40.
[0027] (2) In a 250mL autoclave equipped with mechanical stirring, add 100mL of methanol, 3-aminobutenamide (8.16g, 0.08mol) shown in formula (II-1), 0.4g of Raney nickel, and feed ...
Embodiment 2
[0030] (1) Add 25.0% (mass fraction) ammoniacal liquor (149g, 2.20mol) in the 250mL four-neck round bottom flask that is equipped with mechanical stirring, 3-oxobutanoic acid ethyl ester (13.0 mol) shown in formula (I-2) g, 0.10mol), react at 40°C, and after liquid chromatography detects that the reaction is complete, filter the reaction solution, and the filtrate can be directly applied to the next batch, and the resulting filter cake is soaked with water for 2 to 3 times, and placed in an oven at 50 After drying at ~60°C, 8.55 g of 3-aminobutenamide represented by formula (II-2) was obtained, with a purity of 98.2% as detected by liquid chromatography. Yield 83.8%.
[0031] (2) In a 250mL autoclave equipped with mechanical stirring, add 100mL of methanol, 3-aminobutenamide (8.16g, 0.08mol) shown in formula (II-2), 0.4g of Raney nickel, feed H 2 , keep the pressure 0.5MPa, react under the condition of 40 DEG C, after liquid chromatography detects that the reaction is complet...
Embodiment 3
[0034] (1) Add 37.5% (mass fraction) ammoniacal liquor (136g, 3.00mol) in the 250mL four-necked round bottom flask that is equipped with mechanical stirring, 3-oxophenylbutyric acid ethyl ester shown in formula (I-3) ( 20.6g, 0.10mol), and reacted at 35°C. After the reaction was detected by liquid chromatography, filter the reaction solution. The filtrate can be directly applied to the next batch. The obtained filter cake was soaked with water for 2 to 3 times, and placed in an oven at After drying at 50-60°C, 14.04 g of 3-aminophenylbutenamide represented by the formula (II-3) was obtained, the purity by liquid chromatography was 98.5%, and the yield was 79.8%.
[0035] (2) In a 250mL autoclave equipped with mechanical stirring, add 150mL of methanol, 3-aminophenylbutenamide (12.32g, 0.07mol) shown in formula (II-3), 1.2g of Raney nickel, feed H 2 , keep the pressure 0.5MPa, react under the condition of 45 DEG C, after the liquid chromatography detects that the reaction is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com