Synthetic process of dinotefuran
A synthesis process, the technology of dinotefuran, which is applied in the field of pesticide preparation, can solve the problems of high cost, long synthesis time of dinotefuran, and complicated synthesis process, and achieve the effects of low cost, improved environmental protection effect, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
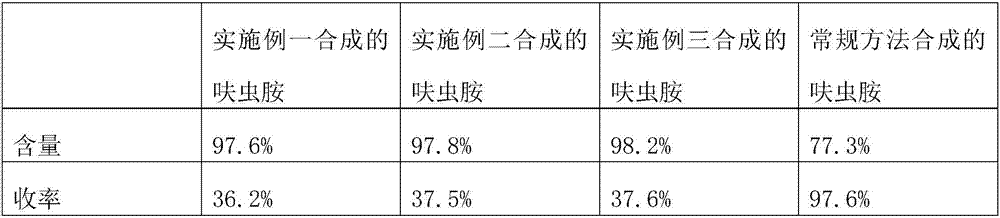
Embodiment 1
[0013] The synthetic technique of this dinotefuran comprises the steps:
[0014] The first step, raw material selection, selects 1,4-butyrolactone as the synthetic raw material for preparing dinotefuran;
[0015] In the second step, the preparation of the intermediate, add 15g of 1,4-butyrolactone, 12g of tribromomethane, 30mL of hydrazine hydrate, and 7g of potassium carbonate in a four-necked reaction flask with a stirrer, a thermometer, and a reflux condenser, and heat Reaction at 70°C for 6 hours. After the reaction is completed, use 12g of diethyl ether to elute. After recovering the diethyl ether, the intermediate 3-(bromomethyl)tetrahydrofuran is obtained, and then add 15g of 3-(bromomethyl)tetrahydrofuran, 12g of lithium hexamethyldisilazide, 6g of potassium carbonate, 6g of sodium sulfate, and 30mL of ethanol were heated to 60°C for 7 hours, and eluted with 12g of diethyl ether after the reaction was completed. After recovering ether, the intermediate 1-(tetrahydro-3...
Embodiment 2
[0018] The synthetic technique of this dinotefuran comprises the steps:
[0019] The first step, raw material selection, selects 1,4-butyrolactone as the synthetic raw material for preparing dinotefuran;
[0020] In the second step, the preparation of the intermediate, add 20g of 1,4-butyrolactone, 13g of tribromomethane, 30mL of hydrazine hydrate, and 8g of potassium carbonate in a four-necked reaction flask with a stirrer, a thermometer, and a reflux condenser, and heat Reaction at 70°C for 7 hours. After the reaction is completed, use 15g of diethyl ether to elute. After recovering the diethyl ether, the intermediate 3-(bromomethyl)tetrahydrofuran is obtained, and then add 20g of 3-(bromomethyl)tetrahydrofuran, 15g of lithium hexamethyldisilazide, 7g of potassium carbonate, 7g of sodium sulfate, and 30mL of ethanol were heated to 60°C for 7 hours, and eluted with 15g of diethyl ether after the reaction was completed. After recovering ether, the intermediate 1-(tetrahydro-3...
Embodiment 3
[0023] The synthetic technique of this dinotefuran comprises the steps:
[0024] The first step, raw material selection, selects 1,4-butyrolactone as the synthetic raw material for preparing dinotefuran;
[0025] In the second step, the preparation of the intermediate, add 30g of 1,4-butyrolactone, 15g of tribromomethane, 30mL of hydrazine hydrate, and 9g of potassium carbonate in a four-necked reaction flask with a stirrer, a thermometer, and a reflux condenser, and heat React at 70°C for 9 hours. After the reaction is completed, use 17g of diethyl ether to elute. After recovering the diethyl ether, the intermediate 3-(bromomethyl)tetrahydrofuran is obtained, and then add 30g of 3-(bromomethyl)tetrahydrofuran, 18g of lithium hexamethyldisilazide, 8g of potassium carbonate, 8g of sodium sulfate, and 30mL of ethanol were heated to 60°C for 7 hours, and eluted with 17g of diethyl ether after the reaction was completed. After recovering ether, the intermediate 1-(tetrahydro-3-fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com