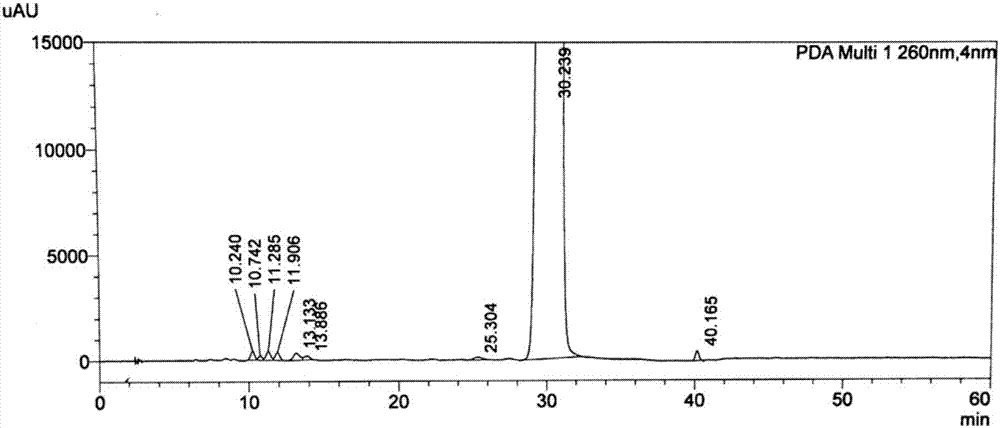
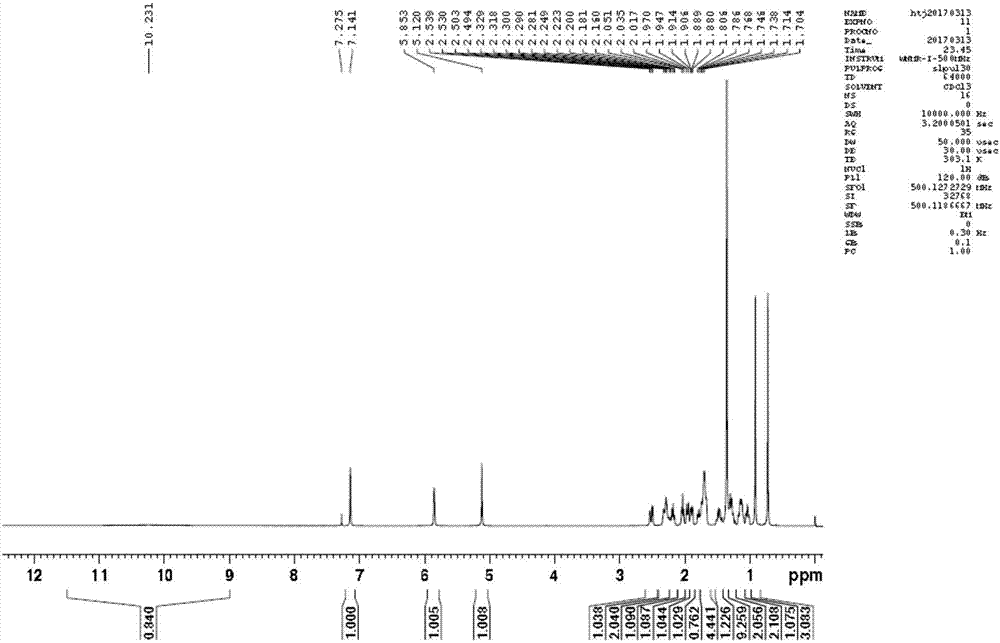
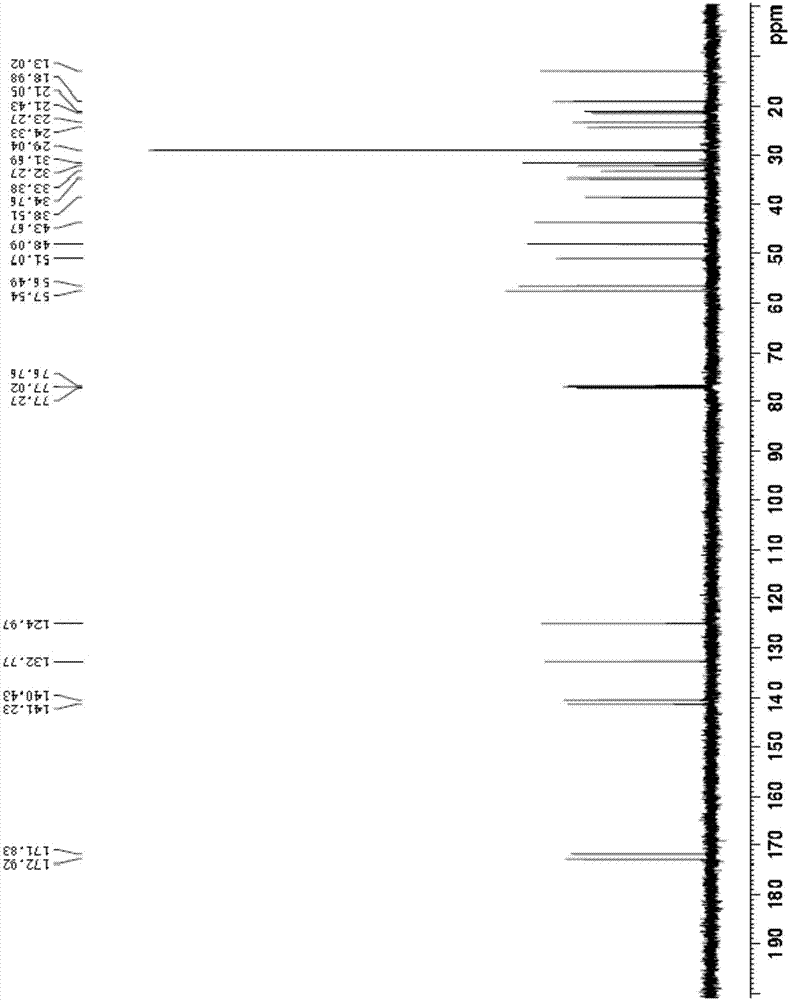
Epristeride intermediate, preparation method of Epristeride intermediate, and preparation method of Epristeride
A technology of Epilite and intermediates, applied in steroids, organic chemistry, etc., can solve the problems of harsh preparation conditions and difficult reagents, and achieve the effect of avoiding column chromatography separation, less side reactions, and easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A preparation method of Apretide intermediate, which comprises the following steps: compound 3 is sulfonated with an alkaline reagent to generate compound 4; the reaction relational formula is as follows:
[0045]
[0046] Compound 3: N-tert-butyl-4-androsten-3-one-17-carboxamide;
[0047] In an embodiment of the present invention, the basic reagent is a non-nucleophilic base, such as: 1,8-diazabicycloundec-7-ene, lithium amide, lithium enolate and 2,6-bis At least one of tert-butyl-4-picoline. Further, the alkaline reagent is 1,8-diazabicycloundec-7-ene and 2,6-di-tert-butyl-4-methylpyridine. Preferably, the basic reagent is 2,6-di-tert-butyl-4-methylpyridine.
[0048] 1,8-Diazabicycloundec-7-ene; Molecular formula: C 9 h 16 N 2 , aka DBU.
[0049] 2,6-Di-tert-butyl-4-methylpyridine; Molecular formula: C 14 h 23 N, also known as DTBMP in English.
[0050] In this example, DTBMP was used as the alkaline reagent.
[0051] In this embodiment, the sulfonated p...
Embodiment 1
[0083] This embodiment provides a kind of Apretide intermediate, which is mainly prepared through the following steps:
[0084] Put compound 3 (3kg) and dichloromethane (24L) into the reaction kettle, drop the temperature to -10~0°C, add DBU (3.6kg), and add trifluoromethanesulfonic anhydride (6.8kg) dropwise at -10~0°C dichloromethane (6L) solution, add and keep warm for 2 hours. TLC tracking detection, when the raw material point disappeared, the reaction solution was quenched by adding 30 L of water, and the layers were separated. Evaporate under pressure to obtain 4.6 kg of dark red oil (Compound 4). The mass yield is 153%.
Embodiment 2
[0086] This embodiment provides a kind of Apretide intermediate, which is mainly prepared through the following steps:
[0087] Put compound 3 (3kg) and dichloromethane (24L) into the reaction kettle, cool down to -20~-10°C, add DBU (3.6kg), keep adding p-toluenesulfonic anhydride (8kg) at -20~-10°C Dichloromethane (6L) solution was added and incubated for 2 hours. TLC tracking detection, when the raw material point disappeared, the reaction solution was quenched by adding 30 L of water, and the layers were separated. Evaporate under pressure to obtain 5.8 kg of dark red oil (compound 4), with a mass yield of 193%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com