Vaccines against avian genetype VII Newcastle disease and preparation method thereof
A genotype and vaccine technology, applied in antiviral agents, viral antigen components, etc., can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
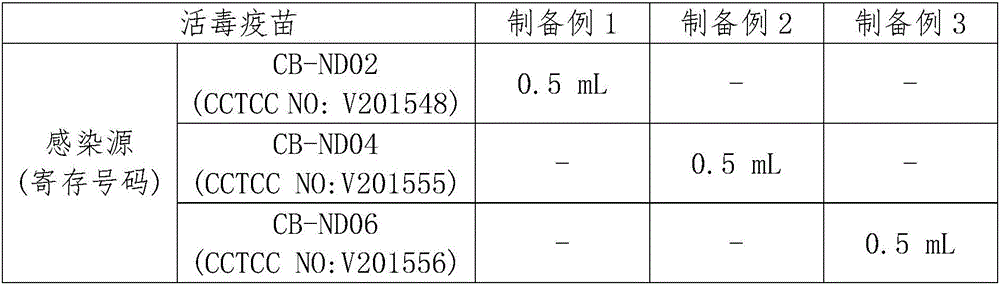
preparation example 1~ preparation example 3
[0061] "Table 1"
[0062]
[0063]
[0064] "Preparation of Deactivated Vaccine"
preparation example 4~ preparation example 6
[0066] First, one of the VII genotype Newcastle disease virus strains (deposited in China Center for Type Culture Collection, deposit numbers CCTCC NO:V201548, CCTCC NO:V201555, CCTCC NO:V201556, The storage date is December 23, 2015) as the source of infection, and then inoculated in the allantoic cavity of 9-day-old specific-pathogen-free (SPF) chicken embryos, or BHK-21 cells as shown in Table 1 (C-13) (ATCC CCL-10) host, and then incubator (Alex Alex electrical processing plant, AI-528) or incubator (Thermo Scientific Forma, Cat.No.311) respectively at 37 ℃ ), and then after 48 hours of culture, the culture fluid was extracted from the above-mentioned chicken embryo allantoic cavity and BHK-21 cells and centrifuged to obtain the VII genotype Newcastle disease antigen solution M4-M6.
[0067] Then, the above-mentioned Genotype VII Newcastle Disease Antigen Solution M4-M6 and Formalin (HT501128-4L, manufactured by Sigma Co.) 20% solution were prepared at a volume ratio of An...
Embodiment 1~ Embodiment 3
[0073] The above-mentioned live virus vaccines VL1~VL3 were administered to immunized 2-3 week-old chickens by nasal and subcutaneous injection respectively, and 6 chickens were administered in each embodiment, and the vaccine dose administered to each chicken was 0.2 ml, blood was collected 3 weeks later to separate serum S1-S24.
[0074] Next, carry out the hemagglutination (HA) test, that is, add 50 μl of HI buffer (3.25g KH 2 P0 4 , 10.8g Na 2 HP0 4 , 170g NaCl, and purified water after three deionized reactions to 2,000mL), and then add 50μl of Genotype VII Newcastle Disease Antigen Solution to the first column.
[0075] Next, after mixing thoroughly with a micropipette, take 50 μl of the Newcastle disease antigen solution of genotype VII in the first row and add it to the second row, and then take another 50 μl and add it to the third row, so that the sequence is diluted to the tenth row, and the antigen presents 2 times to 1024-fold dilution. In row 11 as the contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com