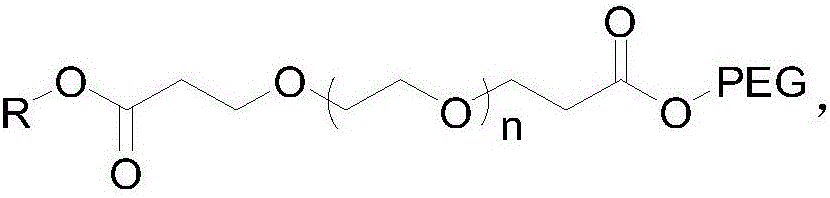Guerbet alcohol nonionic surfactant and preparation method thereof
A Guerbet alcohol, non-ionic surface technology, applied in the field of non-ionic surfactant chemistry, can solve problems such as hindering the reaction, and achieve the effects of good biodegradability, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
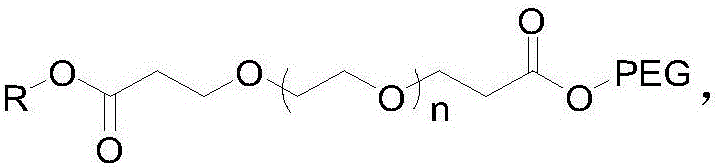
[0025] Guerbet Cetyl Alcohol PEG500 Tetrapolyethylene Glycol Malonate
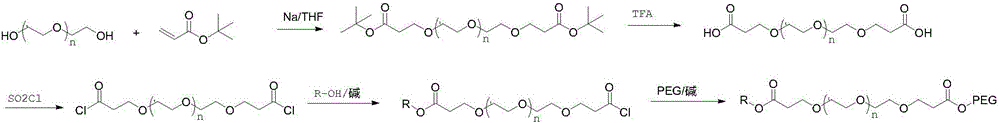
[0026] (1) Add 20 g of tetraethylene glycol into 150 ml of tetrahydrofuran, weigh 0.12 g of crushed sodium metal, add it to the above solution, and stir at room temperature for 1 h. Weigh 33g of tert-butyl acrylate and add dropwise to the above reaction solution, and stir at room temperature for 12h. TLC showed that the reaction was complete, and 20ml of water was added to quench the reaction, the solvent tetrahydrofuran was spin-off, and 150ml of water was added, followed by extraction with 200ml of dichloromethane for 3 times. The dichloromethane phase was dried with anhydrous sodium sulfate, spin-dried, and column purified to obtain about 42 g of polyethylene glycol dipropionate tert-butyl, yield: 92%. NMR data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 3.724(t, J=6.4Hz, 4H); 3.672~3.638(m, 12H); 3.530(t, J=4.8Hz, 4H); 2.893(t, J=6.8Hz, 4H); 1.460 (s, 18H).
[0027] (2) Dissolve 42 g of the product...
Embodiment 2
[0030] Guerbet Cetyl Alcohol PEG2000 Tetrapolyethylene Glycol Malonate
[0031] (1) Add 20 g of tetraethylene glycol to 150 ml of dioxane, weigh 0.15 g of crushed metal potassium, add it to the above solution, and stir at room temperature for 1 h. Weigh 33g of tert-butyl acrylate and add dropwise to the above reaction solution, and stir at room temperature for 12h. TLC showed that the reaction was complete, and 20ml of water was added to quench the reaction, the solvent dioxane was spin-off, and then 150ml of water was added, followed by extraction with 200ml of dichloromethane for 3 times. The dichloromethane phase was dried with anhydrous sodium sulfate, spin-dried, and column purified to obtain 42 g of the product, yield: 92%. NMR data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 3.733(t, J=6.4Hz, 4H); 3.652~3.622(m, 12H); 3.520(t, J=4.8Hz, 4H); 2.890(t, J=6.8Hz, 4H); 1.458 (s, 18H).
[0032](2) Dissolve 42 g of the product obtained in step (1) in 200 ml of dichlorometha...
Embodiment 3
[0035] Guerbet Cetyl Alcohol PEG2000 Tetrapolyethylene Glycol Malonate
[0036] (1) Add 20 g of tetraethylene glycol to 150 ml of dioxane, weigh 0.15 g of crushed metal potassium, add it to the above solution, and stir at room temperature for 1 h. Weigh 33g of tert-butyl acrylate and add dropwise to the above reaction solution, and stir at room temperature for 12h. TLC showed that the reaction was complete, and 20ml of water was added to quench the reaction, the solvent dioxane was spin-off, and then 150ml of water was added, followed by extraction with 200ml of dichloromethane for 3 times. The dichloromethane phase was dried with anhydrous sodium sulfate, spin-dried, and column purified to obtain 42 g of the product, yield: 92%. NMR data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 3.733(t, J=6.4Hz, 4H); 3.652~3.622(m, 12H); 3.520(t, J=4.8Hz, 4H); 2.890(t, J=6.8Hz, 4H); 1.458 (s, 18H).
[0037] (2) Dissolve 42 g of the product obtained in step (1) in 200 ml of dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com