A process for the preparation of methionine alpha-hydroxy analogues from sugars and derivatives thereof
A derivative, methionine technology, applied in thioether preparation, food science, animal feed, etc., can solve the problem of forming regioisomer by-products, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Catalytic reaction in the form of a batch reaction
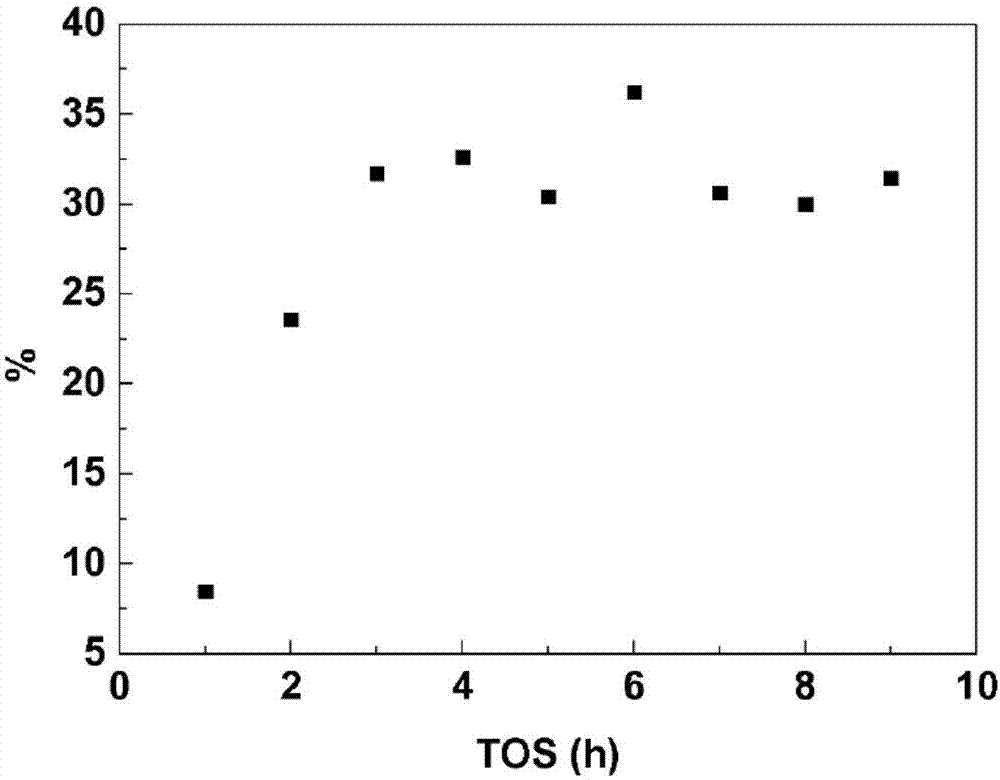
[0046] A stainless steel pressure vessel (40cc, Swagelok) was charged with 15.0 g of methanol (Sigma-Aldrich, >99.8%), 0.450 g of sucrose (Fluka, >99.0%) and 0.150 g of catalyst. Then, the reactor was filled with 75 mL of methyl mercaptan at 1.7 bar and filled with N 2 Pressurize at 11 bar and close. The reactor was heated in an oil bath at 170° C. with stirring (700 rpm). The reaction was allowed to continue for the desired time and after this time the reaction was quenched by immersing the vessel in cold water. A sample from the reaction vessel was filtered and passed through HPLC (Agilent 1200, Biorad Aminex HPX-87H column at 65°C, 0.05M H 2 SO 4 ,0.6ml min -1 ) analysis to quantify unconverted hexoses and dihydroxyacetone (DHA), glyceraldehyde (GLA); and use GC (Agilent 7890 with Phenomenex Solgelwax column) to quantify the following: methyl lactate (ML), vinyl ethanol Methyl 2-hydroxy-3-butenoa...
Embodiment 2
[0056] Example 2: Catalytic reaction in the form of a continuous flow reaction
[0057] available in C 1 -C 3 by biomass or C in the presence of oxygenates 5 -C 6 Pyrolysis of sugars such as glucose, sucrose, fructose or xylose to produce glycolaldehyde-containing compositions. Exemplary pyrolysis reactions are provided in US 7,094,932 B2 and PCT / EP2014 / 053587.
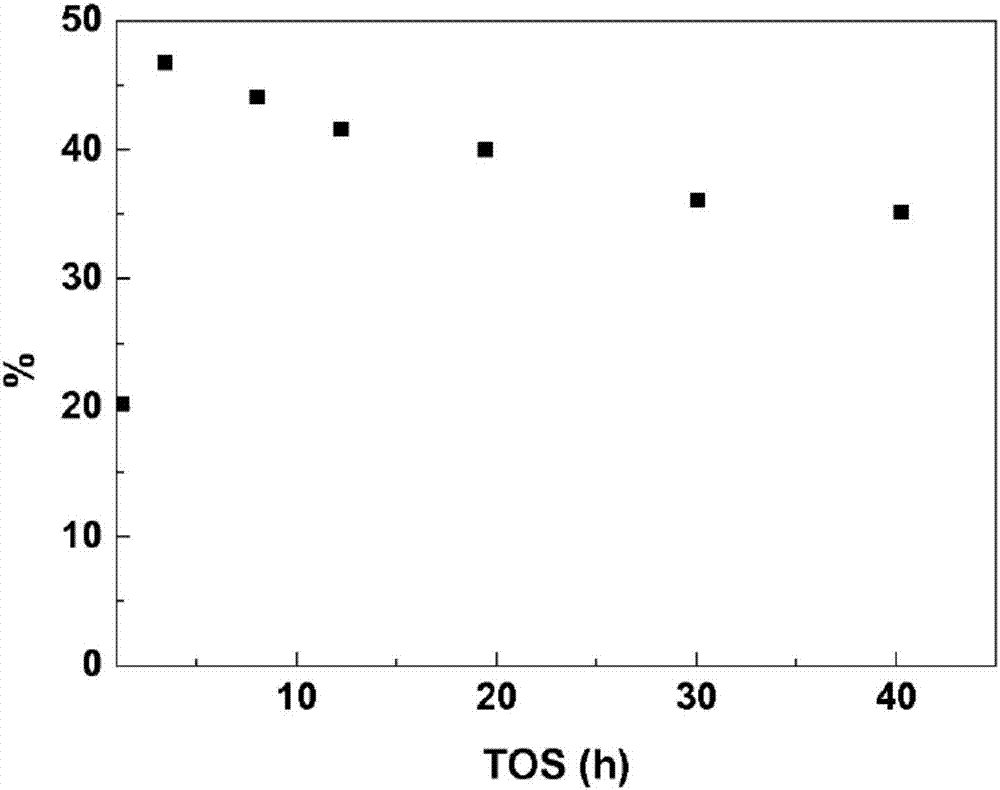
[0058] At room temperature will contain glycolaldehyde or C 1 -C 3The composition of oxygenates was dissolved in methanol (Sigma-Aldrich, 99.9%) together with 814 g / L of glycolaldehyde to achieve a concentration of 10.9 g / l. In addition, methyl mercaptan (Sigma, 1.7 bar) and, if necessary, water, were added to the feed solution. Catalyst Sn-β (Si:Sn125) prepared according to the above preparation process was graded (0.25 g, 300-600 μm.) and loaded into a stainless steel 0.25-inch reactor. Use glass wool to keep the catalyst in place. The reactor was introduced into an oven and the temperature of the reactor...
Embodiment approach 1
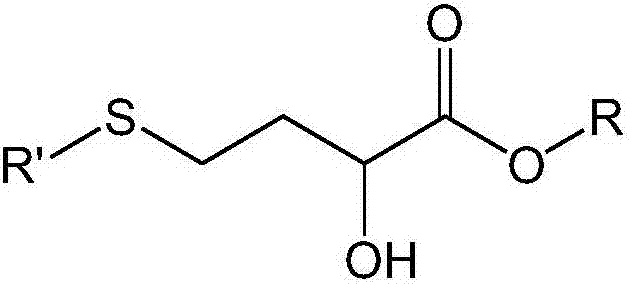
[0062] Embodiment 1. A process for the preparation of methionine alpha-hydroxy analogs comprising contacting one or more sugars or derivatives thereof with a metallosilicate composition in the presence of a sulfur-containing compound and a solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com