Pharmaceutical composition containing amorphous vortioxetine hydrobromide and preparation method thereof
A technology of vortioxetine hydrobromide and composition, which is applied in the field of composition and preparation of vortioxetine hydrobromide and pharmaceutical excipients, which can solve the impact of the release of active pharmaceutical ingredients and the development of pharmaceutical formulations Difficulties and other problems, to achieve industrialized production, avoid the risk of toxic and side effects, and easy to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Any solid form of vortioxetine hydrobromide can be used in the preparation of the pharmaceutical composition of the present invention.
[0046] The calculation method of the load rate of vortioxetine hydrobromide in the pharmaceutical composition is as follows:
[0047] Loading rate=(feeding weight of vortioxetine hydrobromide-the loss weight of vortioxetine hydrobromide in filtrate) / the gross weight of pharmaceutical composition
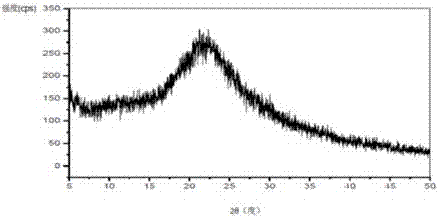
[0048] Vortioxetine hydrobromide (50 mg) and povidone K30 (30 mg) were added to methanol (900 microliters), heated to 60 ° C and stirred to dissolve, then added colloidal silicon dioxide Aerosil 200 ( 30 mg). The above mixture was rapidly cooled to -10°C, a white solid was precipitated, filtered, and dried to obtain 102 mg of a composition of amorphous vortioxetine hydrobromide, povidone K30 and colloidal silicon dioxide Aerosil 200, the active ingredient The load factor is 47.2%. The X-ray powder diffraction pattern of this composition is...
Embodiment 2
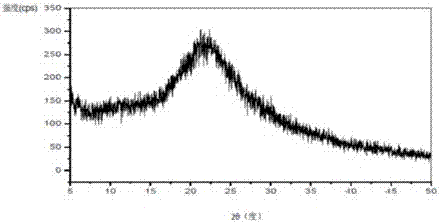
[0050] Vortioxetine (50 mg) and hydroxypropyl methylcellulose HPMC E3 (30 mg) were added to methanol (800 microliters) and dichloromethane (800 microliters), stirred and dissolved at 40 ° C, and then added Colloidal silicon dioxide Aerosil 200 (30 mg). The above mixture was slowly concentrated to dryness in a rotary evaporator, and further vacuum-dried to obtain a white solid, namely 110 mg of a composition of amorphous vortioxetine, hydroxypropylmethylcellulose HPMC E3 and colloidal silicon dioxide Aerosil 200 , the loading rate of the active ingredient was 45.4%. The X-ray powder diffraction pattern of this composition is as follows figure 2 As shown, there is no characteristic peak of the vortioxetine hydrobromide crystal form in the X-ray powder diffraction pattern after deducting the background peak of the pharmaceutical excipient.
Embodiment 3
[0052] Vortioxetine hydrobromide (2 g) and polyethylene glycol 8000 (1.2 g) were added to methanol (50 ml), heated to 60°C and stirred to dissolve, then magnesium aluminum silicate Neusilin UFL2 (0.6 gram). The above mixture was slowly concentrated to dryness in a rotary evaporator, and further vacuum-dried to obtain a white solid, which was further vacuum-dried to obtain a mixture of amorphous vortioxetine hydrobromide, polyethylene glycol 8000 and aluminum magnesium silicate Neusilin UFL2 The composition is 3.8 grams, and the loading rate of the active ingredient is 52.6%. In the X-ray powder diffraction pattern of the composition, there is no characteristic peak of the crystal form of vortioxetine hydrobromide after deducting the background peak of the pharmaceutical excipient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com