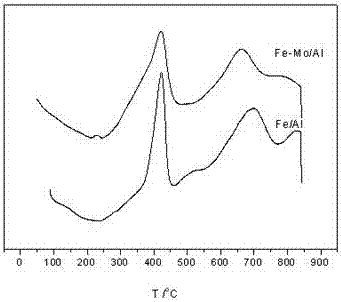
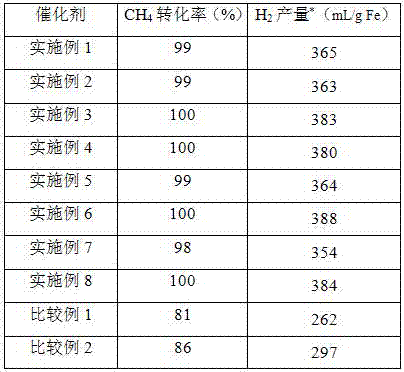
Oxygen carrier for chemical looping hydrogen generation, preparation method and applications thereof
An oxygen carrier and chemical chain technology, applied in the field of chemical chain hydrogen production, can solve the problems of inability to withstand high temperature, limited oxygen carrying rate, and poor cyclic reaction ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 116g, 80~100 mesh Al 2 O 3 The pellets were placed in a rotary evaporator flask, and the temperature of the water bath was maintained at 65°C. Weigh 20g Fe(NO 3 ) 3 ·9H 2 O, 1.3g (NH 4 ) 6 ·Mo 7 O 24 ·4H 2 O, put it into a 500mL beaker, add 100mL deionized water to make a mixed solution, turn on the vacuum pump after dissolving, and suck the mixed solution into the flask, and the rotation speed of the flask is 100r / min. After the solution has evaporated completely, remove the Al 2 O 3The pellets were then dried in a drying oven at 110 °C for 24 hours, and calcined in a muffle furnace at 900 °C for 6 hours to obtain the oxygen carrier Fe 2 O 3 -MoO 3 / Al 2 O 3 , where Fe 2 O 3 The mass content is 20%, MoO 3 The mass content is 5%, Al 2 O 3 The mass content is 75%.
Embodiment 2
[0030] Weigh 116g, 80~100 mesh Al 2 O 3 The pellets were placed in a rotary evaporator flask, and the temperature of the water bath was maintained at 65°C. Weigh 20g Fe(NO 3 ) 3 ·9H 2 O, 6.7g (NH 4 ) 6 ·Mo 7 O 24 ·4H 2 O, put it into a 500mL beaker, add 100mL deionized water to make a mixed solution, turn on the vacuum pump after dissolving, and suck the mixed solution into the flask, and the rotation speed of the flask is 100r / min. After the solution has evaporated completely, remove the Al 2 O 3 The pellets were then dried in a drying oven at 110 °C for 24 hours, and calcined in a muffle furnace at 900 °C for 6 hours to obtain the oxygen carrier Fe 2 O 3 -MoO 3 / Al 2 O 3 , where Fe 2 O 3 The mass content is 20%, MoO 3 The mass content is 20%, Al 2 O 3 The mass content is 60%.
Embodiment 3
[0032] Weigh 116g, 80~100 mesh Al 2 O 3 The pellets were placed in a rotary evaporator flask, and the temperature of the water bath was maintained at 65°C. Weigh 10g Fe(NO 3 ) 3 ·9H 2 O, 1.05g (NH 4 ) 6 ·Mo 7 O 24 ·4H 2 O, put it into a 500mL beaker, add 100mL deionized water to make a mixed solution, turn on the vacuum pump after dissolving, and suck the mixed solution into the flask, and the rotation speed of the flask is 100r / min. After the solution has evaporated completely, remove the Al 2 O 3 The pellets were then dried in a drying oven at 110 °C for 24 hours, and calcined in a muffle furnace at 900 °C for 6 hours to obtain the oxygen carrier Fe 2 O 3 -MoO 3 / Al 2 O 3 , where Fe 2 O 3 The mass content is 10%, MoO 3 The mass content is 5%, Al 2 O 3 The mass content is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com