Preparation method for propranolol
A preparation step, a technology of naphthol, applied in the preparation field of propranolol, can solve the problems of increasing cost and process steps, increasing process steps and costs, and the process is not easy to control, and achieves increasing process steps and costs, and reaction conditions. Not harsh, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of TM
[0036]
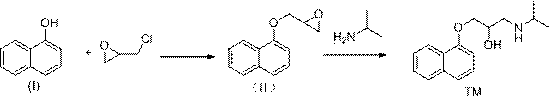
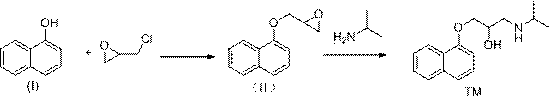
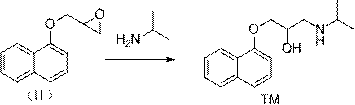
[0037] Naphthol (I) (144.2g, 1mol), epichlorohydrin (113.3, 1.1mol), isopropylamine (90.5g, 1.5mol), tetrabutylammonium bromide (16.6g, 0.05mol) were added to the reaction In the bottle, lower the temperature to about 0°C, then slowly add 10% sodium hydroxide aqueous solution (400g) dropwise to the above reaction flask, after the addition, react at 0°C for 3 hours, then raise the temperature to 100°C and stir the reaction, TLC monitoring After 2 hours of reaction, the temperature was lowered to 0°C, 10% sodium hydroxide aqueous solution (400g) was added dropwise, stirred for 1 hour, filtered, the filter cake was washed with water and drained, dried under reduced pressure, and recrystallized from n-hexane to obtain propranolol (TM ) 233.4 g, yield 90.0%, purity 99.5%.
Embodiment 2
[0039] Naphthol (I) (144.2g, 1mol), epichlorohydrin (113.3, 1.1mol), isopropylamine (90.5g, 1.5mol), tetrabutylammonium bromide (16.6g, 0.05mol) were added to the reaction In the bottle, lower the temperature to about 0°C, then dissolve sodium methoxide (40g) in methanol (360g) and slowly add it dropwise to the above reaction bottle. , TLC monitoring, after 2 hours of reaction, the temperature was lowered to 0°C, 10% sodium hydroxide aqueous solution (400g) was added dropwise, stirred for 1 hour, filtered, the filter cake was washed with water and drained, dried under reduced pressure, and recrystallized from n-hexane to obtain propranol Mole (TM) 230.8 g, yield 89.0%, purity 99.4%.
Embodiment 3
[0041] Add naphthol (I) (144.2g, 1mol), epichlorohydrin (113.3, 1.1mol), isopropylamine (90.5g, 1.5mol), tetrabutylammonium chloride (13.9g, 0.05mol) to In the reaction flask, lower the temperature to about 0°C, then slowly add 10% sodium hydroxide aqueous solution (400g) dropwise to the above reaction flask, after the addition, react at 0°C for 3h, then raise the temperature to 100°C and stir the reaction, TLC Monitoring, after 2 hours of reaction, lower the temperature to 0°C, add 10% sodium hydroxide aqueous solution (400g) dropwise, stir for 1 hour, filter, wash the filter cake with water and drain, dry under reduced pressure, and recrystallize from n-hexane to obtain propranolol ( TM) 228.2 g, yield 88.0%, purity 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com