Method for preparing 1,4-dihydropyridine compounds by utilizing micro-reaction device
A technology of micro-reaction device and dihydropyridine, which is applied in the direction of organic chemistry, can solve the problems of difficult separation of homogeneous catalysts, low degree of automation, active site blocking, etc., and achieve short reaction residence time, high safety, and easy The effect of operational control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
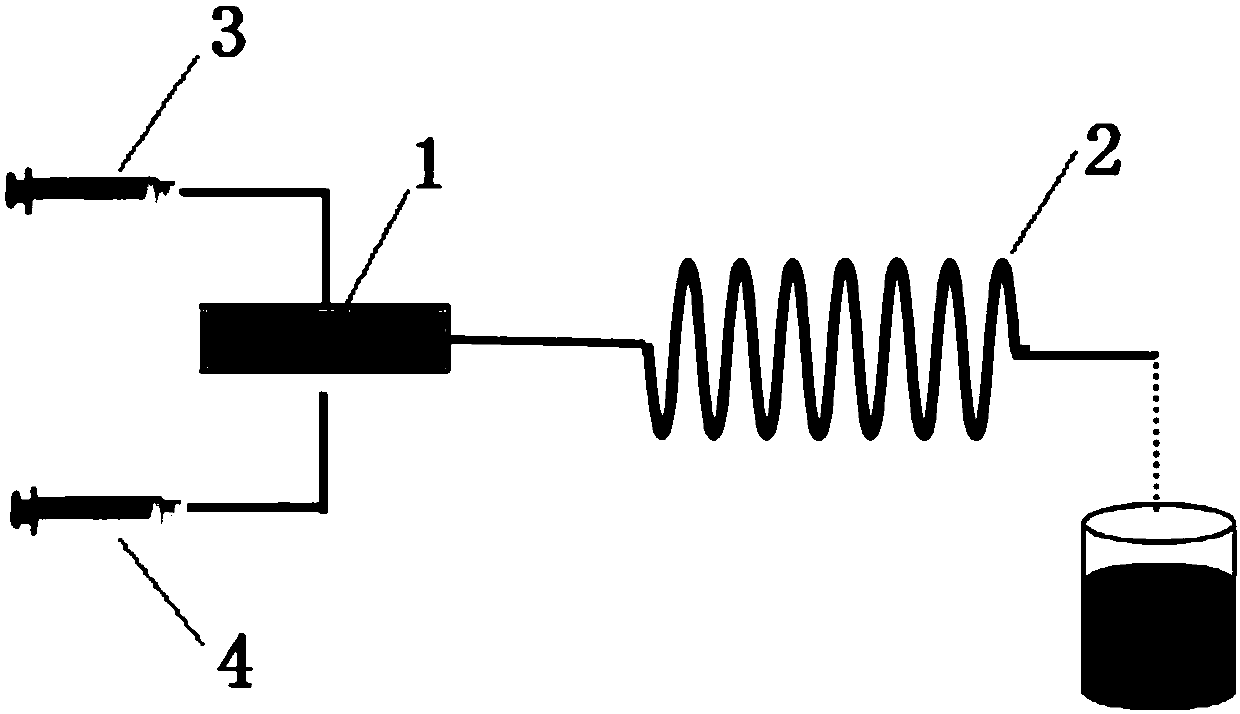
[0026] Dissolve benzaldehyde, ethyl acetoacetate and ammonium acetate into a homogeneous phase (wherein the molar ratio of benzaldehyde: ethyl acetoacetate: ammonium acetate = 1:2:1.5) is recorded as solution A, and the particle size is 10nm γ- Fe 2 o 3 Nanoparticles dispersed in absolute ethanol (where γ-Fe 2 o 3 The concentration of nanoparticle is 250mg / mL) is recorded as solution B; Then solution A and solution B are mixed through micro-mixer, wherein solution A adopts HPLC pump to inject in the micro-mixer, and solution B adopts slurry pump to inject in the micro-mixer, γ-Fe 2 o 3 The molar ratio of nanoparticles to aromatic aldehyde was 0.05:1, mixed well and injected into the microreactor (the inner diameter of the capillary of the microreactor was 1mm, and the capillary volume was 5mL) and reacted at 120°C for 5min. The output of the microreactor is centrifuged to obtain an organic phase, and the organic phase is washed with Na 2 CO 3 After alkali washing and di...
Embodiment 2
[0029] Dissolve benzaldehyde, ethyl acetoacetate and ammonium acetate into a homogeneous phase (the molar ratio of benzaldehyde: ethyl acetoacetate: ammonium acetate is 1:3:2) and record it as solution A, the particle size is 10nm gamma-Fe 2 o 3 Nanoparticles dispersed in absolute ethanol (where γ-Fe 2 o 3The concentration of nanoparticles is 350 mg / mL) as solution B; then solution A and solution B are mixed through a micro-mixer, wherein solution A is injected into the micro-mixer using an HPLC pump, and solution B is injected into the micro-mixer using a slurry pump , γ-Fe 2 o 3 The molar ratio of nanoparticles to aromatic aldehyde was 0.1:1, mixed well and injected into the microreactor (the inner diameter of the capillary of the microreactor was 1mm, and the capillary volume was 7mL) and reacted at 130°C for 7.5min. The output of the microreactor is centrifuged to obtain an organic phase, and the organic phase is washed with Na 2 CO 3 After alkali washing and distill...
Embodiment 3
[0032] Dissolve benzaldehyde, ethyl acetoacetate and ammonium acetate into a homogeneous phase (wherein the molar ratio of benzaldehyde: ethyl acetoacetate: ammonium acetate is 1:4:3) is recorded as solution A, and the particle size is 50nm gamma-Fe 2 o 3 Nanoparticles dispersed in absolute ethanol (where γ-Fe 2 o 3 The concentration of nanoparticles is 450 mg / mL) as solution B; then solution A and solution B are mixed through a micro-mixer, wherein solution A is injected into the micro-mixer using an HPLC pump, and solution B is injected into the micro-mixer using a slurry pump , γ-Fe 2 o 3 The molar ratio of nanoparticles to aromatic aldehyde was 0.15:1, mixed well and poured into the microreactor (the inner diameter of the capillary of the microreactor was 1mm, the capillary volume was 9mL) and reacted at 140°C for 10min. The output of the microreactor is centrifuged to obtain an organic phase, and the organic phase is washed with Na 2 CO 3 After alkali washing and di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com