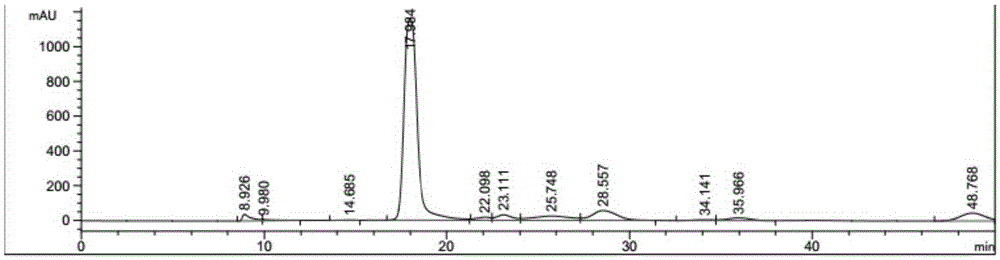
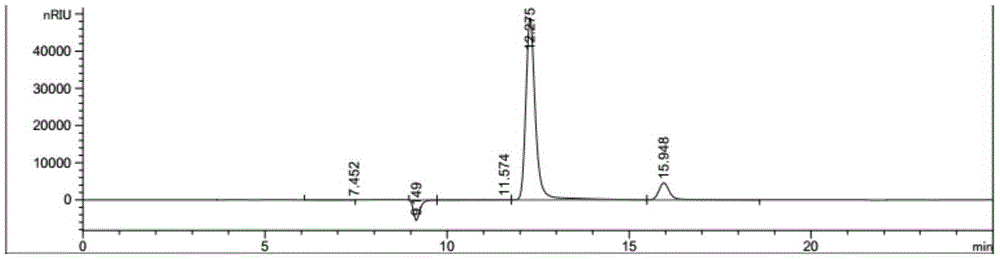
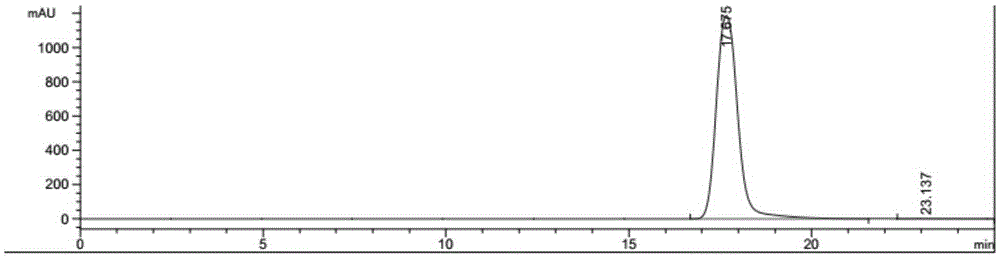
Method for preparing 2,5-furandicarboxylic acid by supported bifunctional catalyst by catalyzing fructose
A bifunctional catalyst, furandicarboxylic acid technology, applied in molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as inconsistency of catalysts, easy generation of waste water, strong corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] K 6 [CoW 12 o 40 ]·16H 2 The preparation of O solid heteropolyacid:
[0046] (A1) 19.8gNa 2 WO 4 2H 2 O was dissolved in 40mL deionized water and adjusted to pH5.0-7.0 with glacial acetic acid to obtain solution A, 2.5g (CH 3 CO 2 ) 2 Co 4H 2 O was dissolved in 12mL deionized water, adjusted to pH 5.0-7.0 with glacial acetic acid to obtain solution B; both solution A and solution B were heated to close to the boiling point, and solution B was slowly dropped into solution A while hot to produce a dark green solution , the mixture was boiled for 10-20 minutes and then filtered while it was hot;
[0047] (A2) Reheat the filtrate obtained in step (A1), and dissolve 20 mL of saturated CH with a pH of 6.0-7.0 3 Slowly add COOK solution to the heated filtrate, stir to produce a green solid, cool to room temperature and filter, wash the solid twice with the filtrate, dissolve the solid in 40mL of 2M sulfuric acid solution, stir at 50°C for 15min, the solution turns d...
Embodiment 2
[0049] Solid catalyst heteropoly acid salt K 5 [Co III W 12 o 40 ]·20H 2 Preparation of O:
[0050] On the basis of Example 1, the filtrate C was heated to boiling, and 10 g K 2 S 2 o 8 , after the solution turns from blue to yellow, stop heating, cool in an ice bath to obtain yellow needle-like crystals, filter to obtain K 5 [Co III W 12 o 40 ]·20H 2 O solid heteropolyacids.
Embodiment 3
[0052] Dawson type Co 2 h 6+n P 2 Mo 18-n V n o 62 (n=3) preparation of heteropolyacid catalyst:
[0053] (A1) Dissolve 0.015mol of ammonium metavanadate in 75ml of distilled water, dissolve 0.01mol of sodium dihydrogen phosphate in 12.5ml of distilled water, stir to make it fully dissolved and mix, add dropwise 49wt% sulfuric acid aqueous solution to adjust pH4.0-6.0, add 0.075mol Sodium molybdate aqueous solution 37.5ml, add 49wt% sulfuric acid aqueous solution dropwise again to adjust pH 4.0-6.0, reflux at 100-120°C for 8h, cool, extract with ether, add a small amount of 49wt% sulfuric acid aqueous solution in batches, shake until red oil appears , standing for stratification, separating the lower layer, standing in the fume hood for 1-2 days, until the crystalline particles are precipitated, adding a small amount of distilled water to recrystallize, and vacuum drying to obtain phosphomolybdovanadate heteropolyacid H 9 P 2 Mo 15 V 3 o 62 ;
[0054] (A2) 2mmol of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com