Preparation method of imrecoxib, and preparation method of imrecoxib intermediate
A compound and condensing agent technology, applied in the field of preparation of Erecoxib and its intermediates, can solve problems such as strong irritation, low yield, physical health of production personnel and environmental damage, and achieve improved yield and post-processing simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
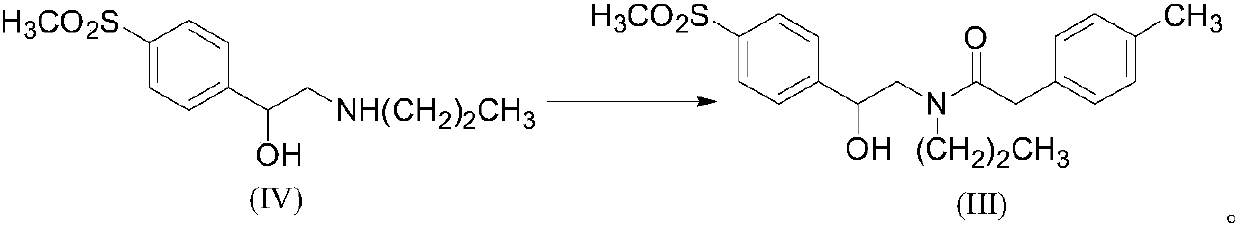
[0031] Example 1: Preparation of the compound N-n-propyl-N-[2-hydroxyl-2-(4'-methylsulfonylphenyl)]ethyl-4-methylphenylacetamide (III)
[0032] 1) Using SOCl 2 preparation
[0033] Add 10g (0.067mol) p-methylphenylacetic acid and 40ml SOCl in a 100ml three-necked bottle 2 Heating to reflux for 1.5h, distilling off SOCl 2 , add 50ml tetrahydrofuran, obtain the tetrahydrofuran solution of p-methylphenylacetyl chloride, add dropwise the solution of 17.21g (0.067mol) N-n-propyl-beta-hydroxyl-4-methylsulfonylphenylethylamine dissolved in tetrahydrofuran and 16ml of pyridine, continue to react for 1h, the reaction is complete, evaporate the solvent, and dissolve the residue in 50ml of dichloromethane, wash with 1N hydrochloric acid, saturated sodium bicarbonate solution and saturated brine respectively, and dry with anhydrous sodium sulfate, concentrate under reduced pressure and evaporate The solvent was removed to obtain 15.9 g of compound (III), with a yield of 61% and a purit...
Embodiment 2
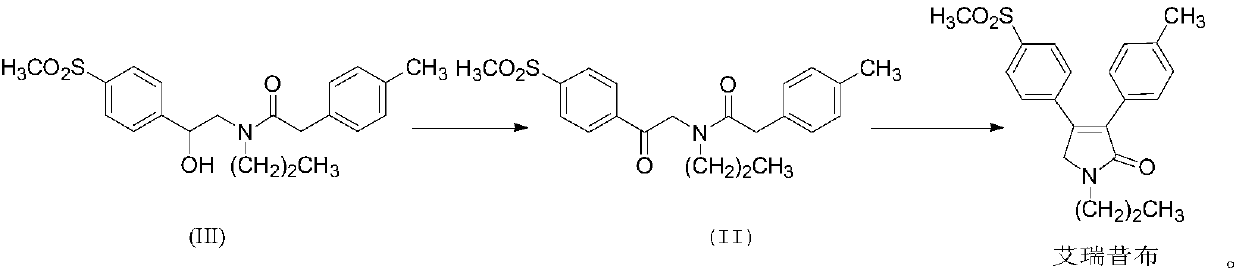
[0039] Embodiment 2: Preparation of Erecoxib
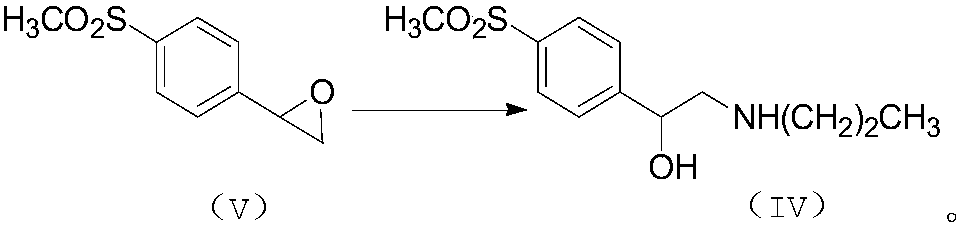
[0040] Step 1): Preparation of N-n-propyl-β-hydroxyl-4-methylsulfonylphenethylamine (IV)
[0041] Put 10.0kg of compound (V) and 236kg of anhydrous methanol into a 500L reactor, heat to dissolve and cool to below 5°C, add 1.0kg of neutral alumina (100-200 mesh), continue to slowly add 29.6 kg n-propylamine, seal the reaction kettle, pass through frozen brine and keep stirring at 0-10°C for 5 days, after the reaction is complete, filter, evaporate the reaction solution under reduced pressure to obtain 13kg of light yellow solid, add 32kg of dichloromethane to dissolve, and continue to add 88kg Ethyl acetate / petroleum ether mixture (ethyl acetate / petroleum ether=1:2), let stand, filter, and dry to obtain 9.4kg of compound (IV), with a yield of 72%.
[0042] Compound (IV): 1 H NMR (CDCl 3 )δ (ppm): 7.92 (d, 2H, ArH); 7.59 (d, 2H, ArH); 4.77 (dd, 1H, CH); 3.05 (s, 3H, CH 3 SO 2 ); 2.97 (dd, 1H, CH 2 ); 2.63 (dd, 1H, CH 2 ); 2.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com