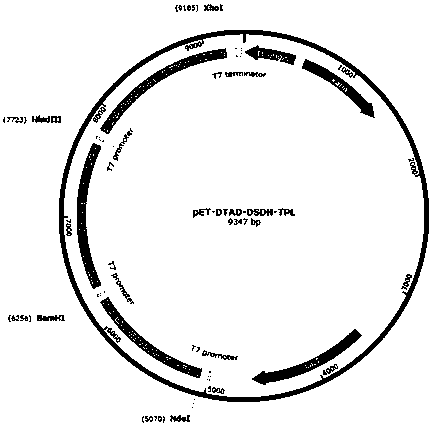
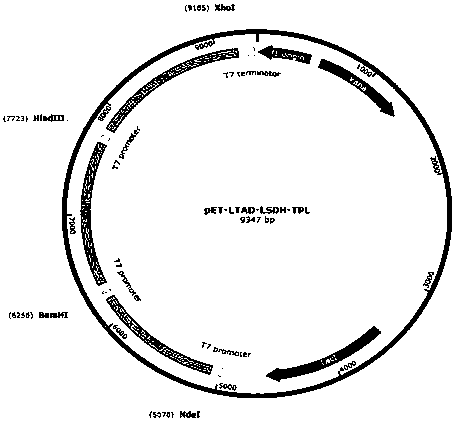
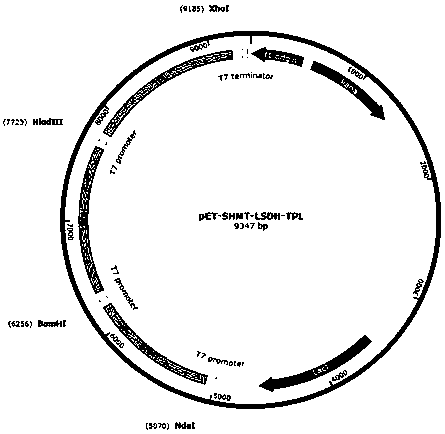
Method for preparing levodopa with one-pot enzymatic method
A technology for levodopa and enzymatic preparation, which is applied in the field of bioengineering, can solve the problems of serious environmental pollution and high production cost, and achieves the effects of high yield, low production cost and low environmental protection pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the conversion of 2.5 tons of systems
[0072] Add 1600L of water, 120kg of glycine, and 55kg of formaldehyde solution (37%) in the bioreactor, and use concentrated ammonia to control the pH at 6.0 to 8.0; add 0.33kg of pyridoxal 5-phosphate, and use concentrated ammonia to control the pH At 6.0-8.0, the temperature is controlled at 32°C-38°C. Add 25kg of catechol and 650kg of enzyme solution containing recombinant D-threonine aldolase, recombinant D-serine dehydratase and recombinant tyrosine phenolase to start the reaction. During the reaction process, 37% formaldehyde solution was added to maintain the reaction pH between 6.0 and 8.0. After that, 5kg of substrate catechol was added every 10min. The total input amount of catechol is 176kg. After the catechol feeding was finished, the reaction was continued for 2 hours, and the sample was taken for detection. The concentration of levodopa was 67g / L, and the conversion rate was about 53.2%. After post-...
Embodiment 2
[0073] Embodiment 2: the transformation of 2.5 tons system
[0074]Add 1600L of water, 140kg of glycine, 62.5kg of formaldehyde solution (37%) to the bioreactor, and control the pH at 6.0 to 8.0 with concentrated ammonia water; then add 5kg of sodium sulfite, 2.5kg of EDTA, 50kg of ammonium acetate, 5 -Pyridoxal phosphate 0.33kg, again use concentrated ammonia water to control the pH at 6.0-8.0, and control the temperature at 32°C-38°C. Add 25kg of catechol and 600kg of enzyme solution containing recombinant D-threonine aldolase, recombinant D-serine dehydratase and recombinant tyrosine phenolase to start the reaction. During the reaction process, 37% formaldehyde solution was added to maintain the reaction pH between 6.0 and 8.0. After that, 5kg of substrate catechol was added every 10min. The total input amount of catechol is 205kg. After the catechol feeding was finished, the reaction was continued for 1 hour, and the sample was taken for detection. The concentration of ...
Embodiment 3
[0075] Embodiment 3: the transformation of 7.5 tons system
[0076] Add 4800L of water, 450kg of glycine, and 180kg of formaldehyde solution (37%) to the bioreactor in sequence, and control the pH at 6.0 to 8.0 with concentrated ammonia water; then add 15kg of VC, 7.5kg of EDTA, 120kg of ammonium formate, Pyridoxal 1kg, again use concentrated ammonia water to control the pH at 6.0-8.0, and control the temperature at 32°C-38°C. Add 75kg of catechol and 1800kg of enzyme solution containing recombinant L-threonine aldolase, recombinant L-serine dehydratase and recombinant tyrosine phenolase to start the reaction. During the reaction process, 37% formaldehyde solution was added to maintain the reaction pH between 6.0 and 8.0. After that, 15kg of substrate catechol was added every 10min. The total input amount of catechol is 660kg. After the catechol feeding was finished, the reaction was continued for 1.5h, and the sample was taken for detection. The concentration of levodopa w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com