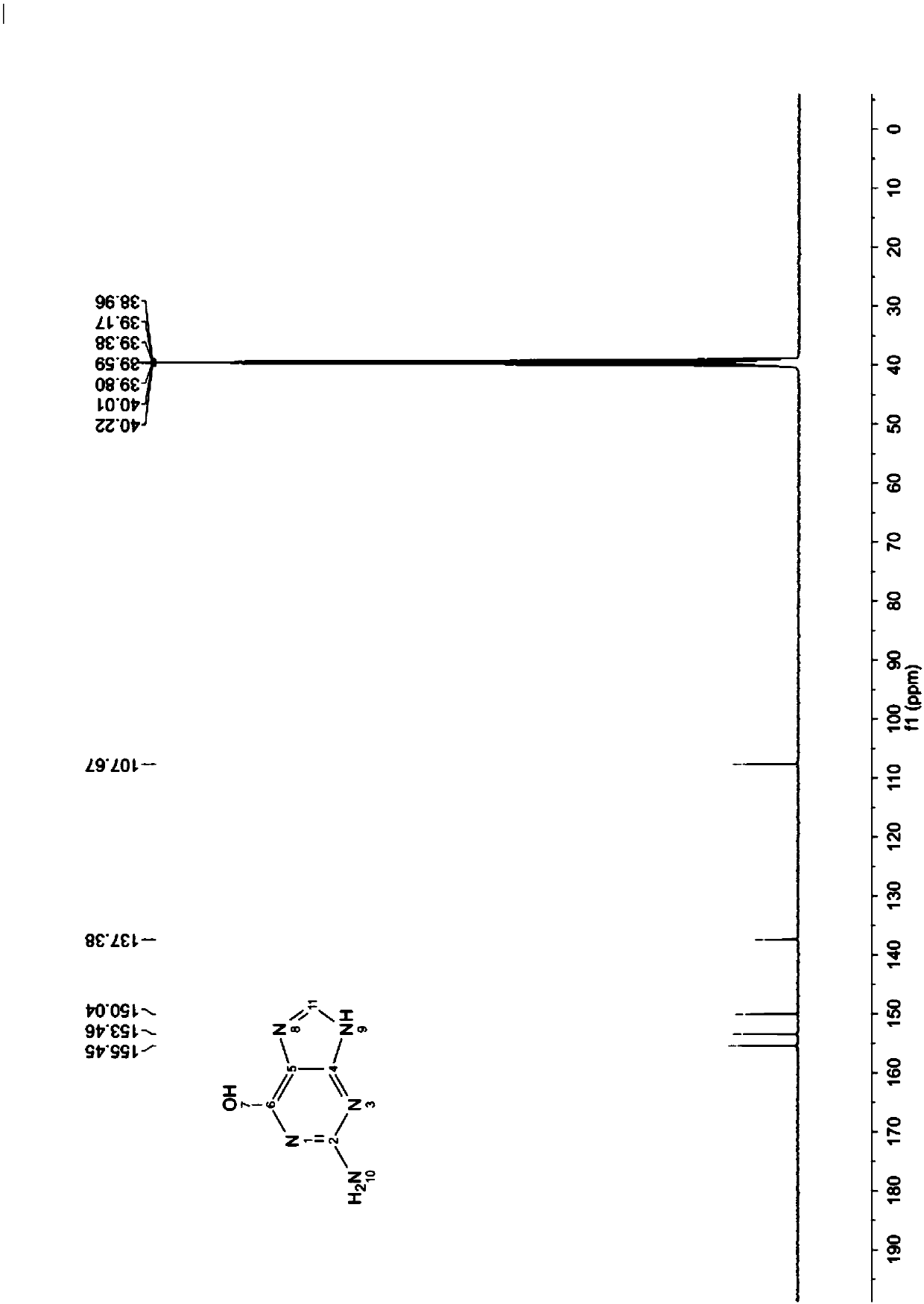
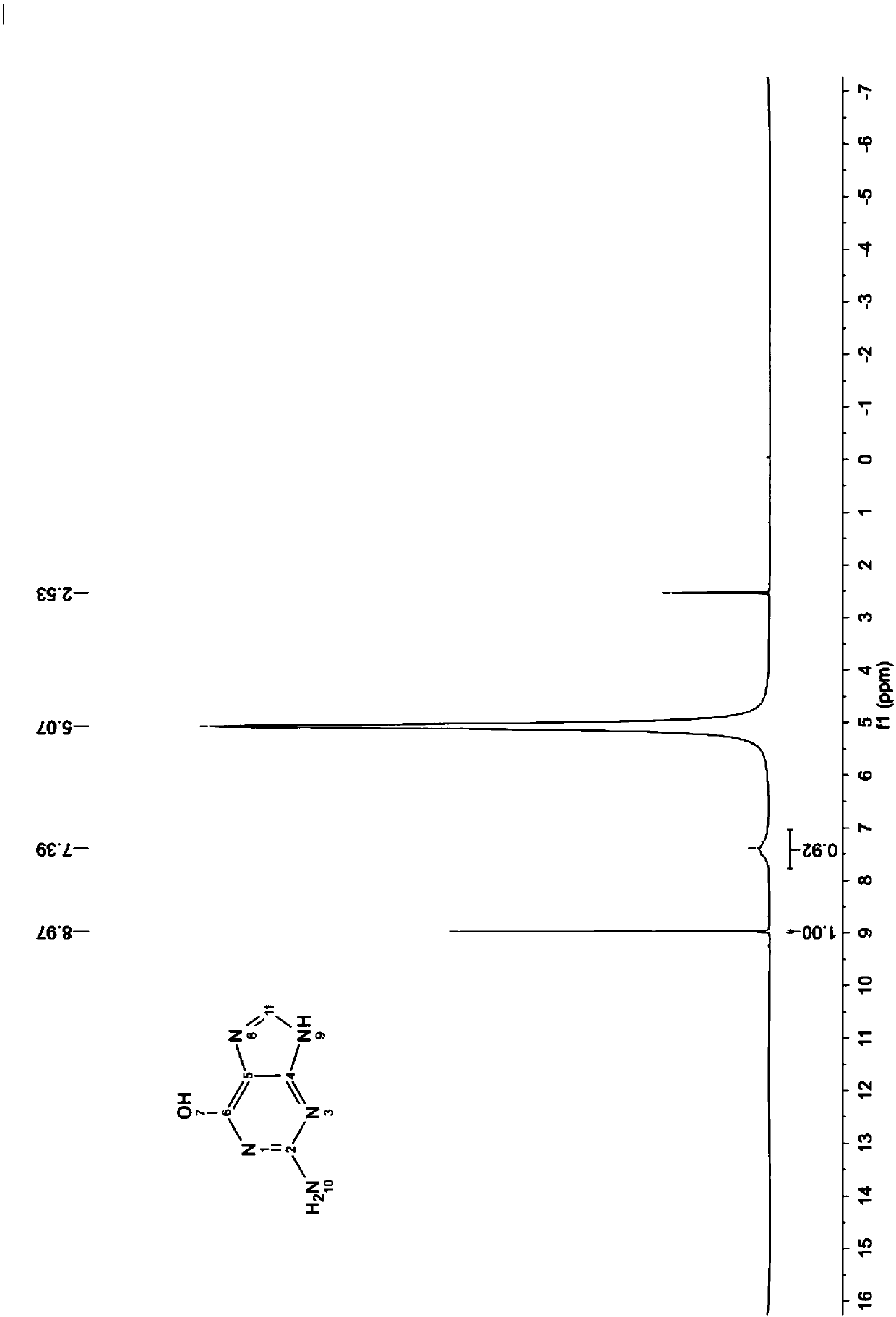
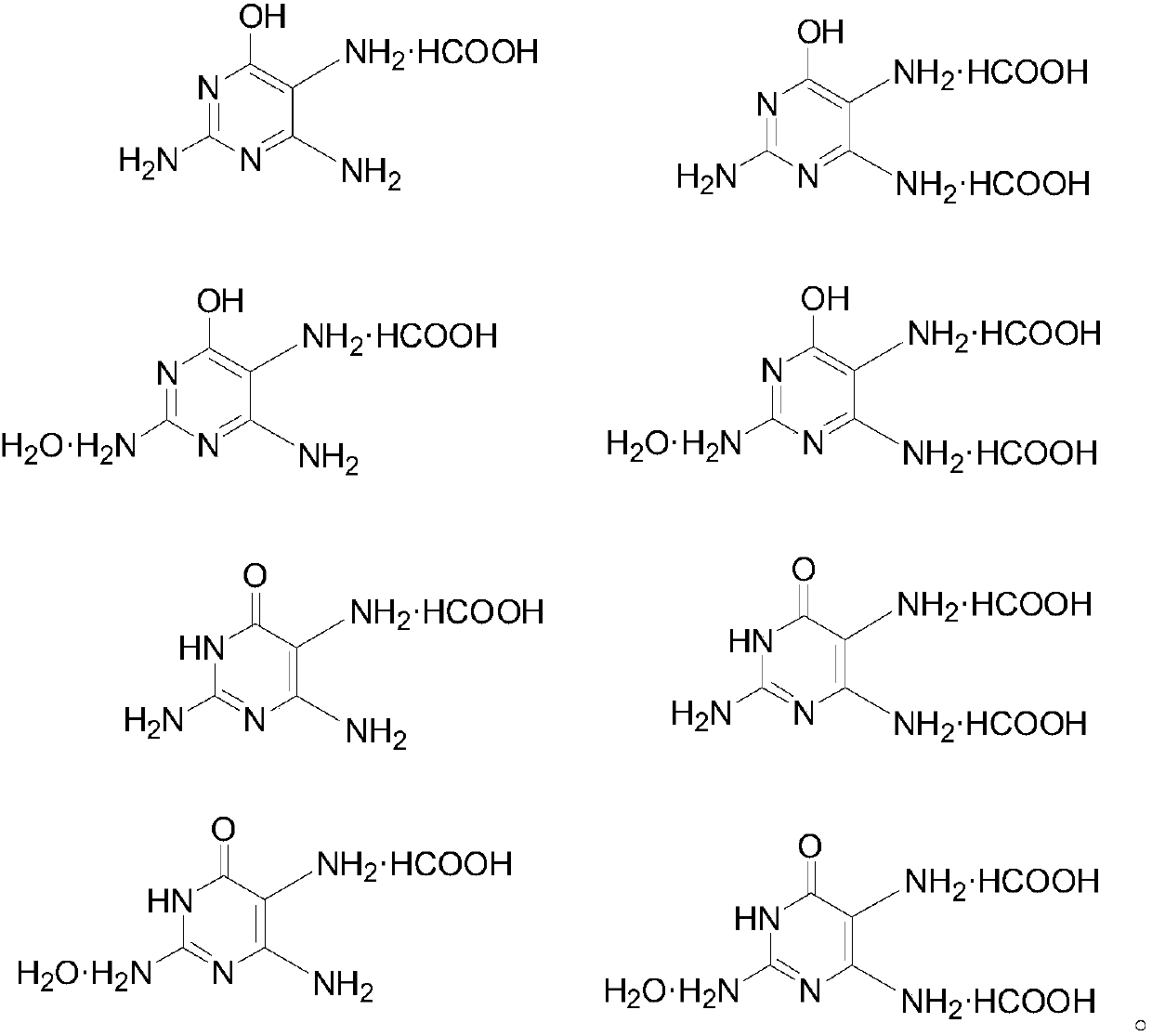
2,4,5-triamido-6-hydroxy pyrimidine formate, preparation method and application
A hydroxypyrimidine and triamino technology, which is applied in the fields of 2,4,5-triamino-6-hydroxypyrimidine formate, preparation and application, can solve the problems of high salt, unfriendly environment, etc. The effect of short production route and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Mix 155g of 2,4-diamino-5-nitroso-6-hydroxypyrimidine (hereinafter referred to as "nitroso", MW155.11, 1.00mol) with 1800mL drinking water, make a certificate, and pump 3L Hydrogenation kettle. Turn on the hydrogenation tank and stir, and inhale 160g of 30% lye (sodium hydroxide aqueous solution, 1.2mol) and 18g of W-type Raney nickel.
[0057] After feeding, fill the air with high-purity nitrogen and replace it 3 times; then replace it with hydrogen 3 times.
[0058] After the replacement, pressurize with hydrogen to 0.5-0.6MPa, heat and raise the temperature to 50-60°C. Keeping warm, keeping the pressure, and stirring rapidly until the hydrogen is no longer absorbed (that is, the hydrogen pressure in the kettle does not drop within 30 minutes).
[0059] In advance, 380 g (MW46.03, 4.13 mol) of formic acid solution with a mass percent concentration of 50% was added into a 3L three-necked flask, replaced with nitrogen for 3 times, stirred and protected by nitrogen, an...
Embodiment 2
[0068] Mix 155g of 2,4-diamino-5-nitroso-6-hydroxypyrimidine (hereinafter referred to as "nitroso", MW155.11, 1.00mol) with 1500mL of drinking water, make a slurry, and pump it into 3L hydrogenation kettle. Open the hydrogenation tank and stir, inhale 200g of 30% lye (1.5mol) and 18g of W-type Raney nickel.
[0069] After feeding, fill the air with high-purity nitrogen and replace it 3 times; then replace it with hydrogen 3 times.
[0070] After the replacement, pressurize with hydrogen to 0.6-0.8MPa, heat and raise the temperature to 50-60°C. Heat preservation, pressure maintenance, and rapid stirring until no hydrogen is absorbed.
[0071] In advance, 300 g (MW46.03, 3.26 mol) of 50% formic acid was added into a 3L three-necked flask, replaced with nitrogen for 3 times, stirred, and kept under nitrogen protection for use.
[0072] After the hydrogenation reaction, the excess hydrogen in the kettle was drained, and then replaced with high-purity nitrogen for 3 times; then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com