Ondansetron water-free-swallowing granules
A technology of ondansetron and granules is applied in the field of ondansetron anhydrous swallowing granules, which can solve the problem that the raw material drug ondansetron tastes bitter and numb, the production cost and storage conditions of injections are relatively high, and the Patients take pain and other problems, and achieve the effects of solving the degradation of ondansetron, solving the bitter taste, and solving the inconvenience of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
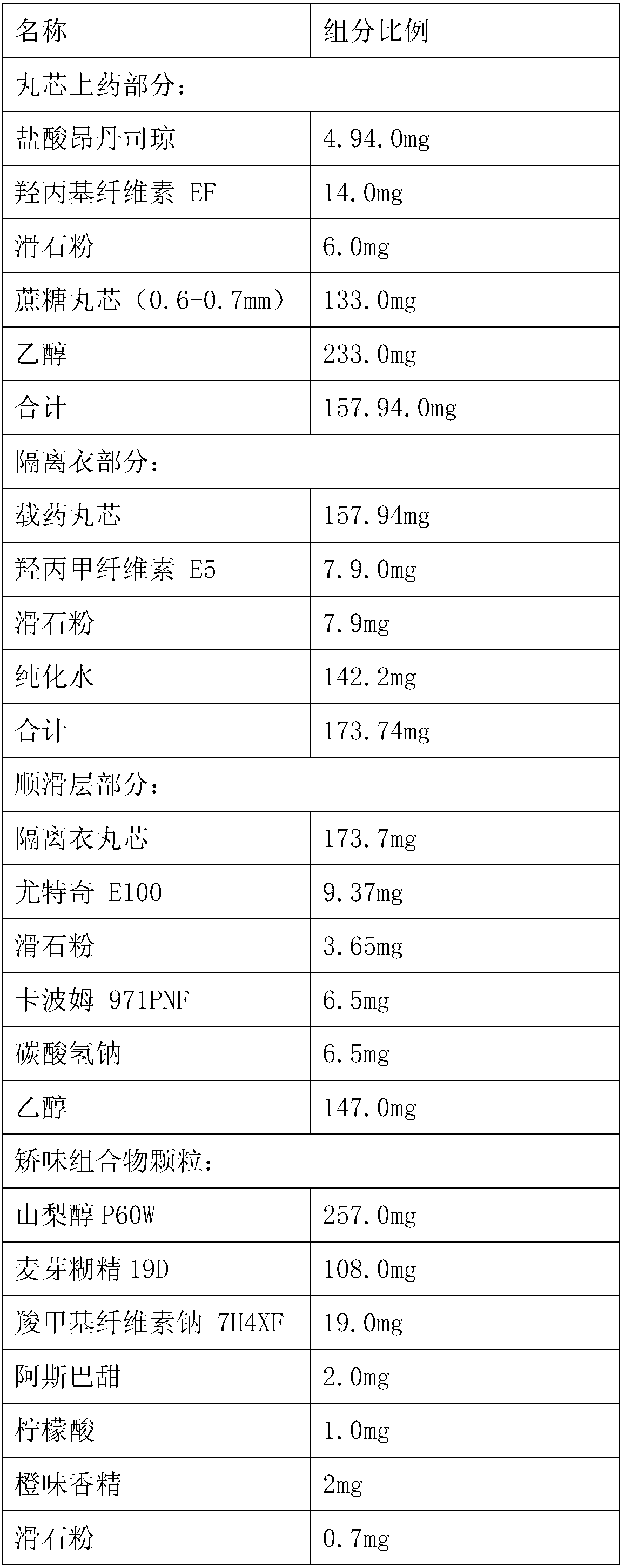
[0054] Ondansetron granules in the amount of 6000 unit preparation:
[0055]
[0056]
[0057] Preparation Process:
[0058] 1. Pill core application—raw and auxiliary materials include: ondansetron hydrochloride, hydroxypropyl cellulose, talcum powder, and ethanol to prepare a drug-containing suspension for pill core application.
[0059] Drug-containing suspension preparation:
[0060]
[0061] Process: adopt the fluidized bed medicine process to prepare ondansetron-coated pill cores, and control the temperature of the material at 35-40°C. After preparation, take the pill cores between 20-30 mesh sieves for later use to prepare drug-containing pill cores.
[0062] 2. Isolation layer packaging - raw and auxiliary materials include: hydroxypropyl methylcellulose, talc powder, purified water, prepared into an isolation layer suspension for isolation layer packaging.
[0063] Preparation of the isolation layer coating suspension:
[0064]
[0065] Process: adopt t...
Embodiment 2
[0081] Influence of isolation gown weight gain (4%, 6%, 10%) on sample dissolution and related substances
[0082]
[0083]
[0084] Preparation technology is the same as embodiment 1
[0085] Isolation gown weight gain test sample dissolution results:
[0086]
5min
15min
30min
4%
54.9%
80.3%
94.4%
6%
52.1%
81.3%
93.2%
10%
51.6%
82.1%
93.8%
[0087] Conclusion: The weight gain of the gown between 4% and 6% has no obvious effect on the dissolution rate of the sample.
[0088] The influence of different weight gain of isolation gowns on the growth trend of related substances in the samples was investigated. The weight gain of the gowns was 4%, 6%, and 10%. The samples were placed at 40°C and 75% Rh for 60 days. After 15 days, 30 days, and 60 days, take samples and compare the changing trend of related substances in the samples.
[0089] The result is as follows:
[0090]
[0091] in conclusion: ...
Embodiment 3
[0095] The impact of smooth layer weight gain (7%, 10%, 15%) on sample dissolution and related substances
[0096]
[0097] Preparation technology is the same as embodiment 1
[0098] Smooth layer weight gain to investigate the dissolution rate results of samples:
[0099]
5min
15min
30min
7%
55.9%
83.3%
93.4%
10%
53.1%
81.3%
95.2%
15%
51.5%
85.1%
94.8%
[0100] Conclusion: 1. The weight gain of the smooth layer of the sample is between 7% and 15%, which has no obvious influence on the dissolution rate of the sample.
[0101] 2. The weight gain of the smooth layer of the comparative sample is between 7% and 15%, and the taste of the sample is swallowed. With the increase of the weight gain of the smooth layer, the smooth feeling of the sample in the oral cavity increases.
[0102] 3. The weight gain of the smooth layer of the sample is between 7% and 15%, and the dissolution and swallowing effect of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap