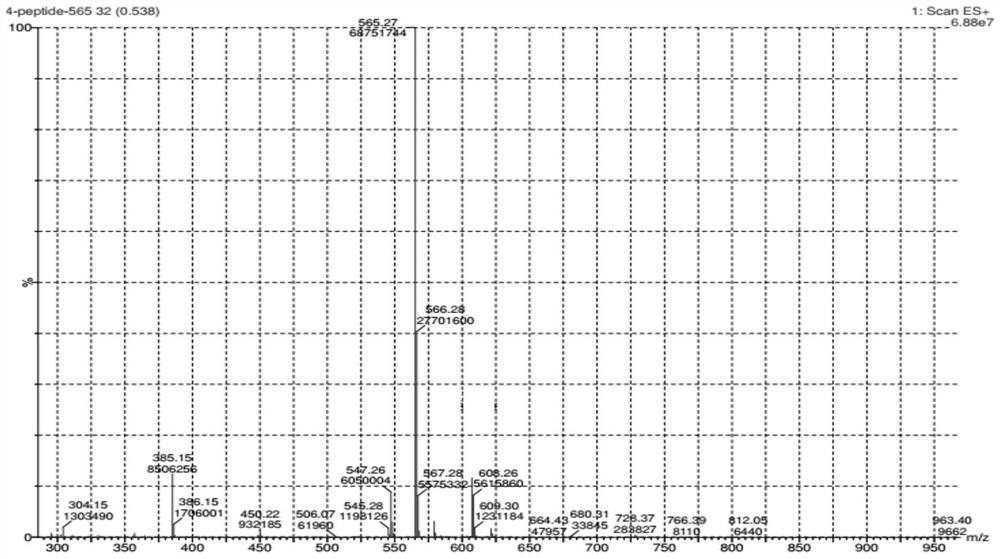
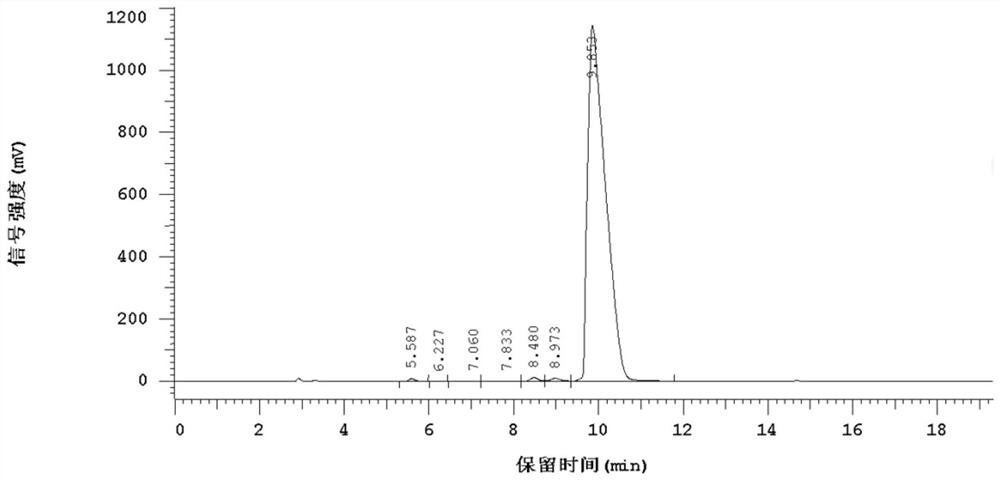
A kind of liquid phase synthesis method of acetyl tetrapeptide-2
A technology of acetyl tetrapeptide and liquid phase synthesis, which is applied to the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problem of high synthesis cost, and achieve the effect of simple synthesis method, reduced production cost, and reduced synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0035] (1) Synthesis of Ac-D-Lys(Boc)-OH
[0036] Mix and dissolve 24.6g (100mmol) H-D-Lys(Boc)-OH with 180mL dichloromethane and 8.7mL (50mmol) N,N'-diisopropylethylamine, then add 11.3mL (120mmol) acetic anhydride, room temperature React for 1 hour; concentrate the reaction liquid, add diethyl ether to make a slurry, filter, wash the filter cake, and dry under vacuum at room temperature to obtain 17.9 g of Ac-D-Lys(Boc)-OH.
[0037] (2) Synthesis of Ac-D-Lys(Boc)-COOSu
[0038] Mix 17.9g (95mmol) Ac-D-Lys(Boc)-OH, 200mL tetrahydrofuran and 12.0g (105mmol) N-hydroxysuccinimide until dissolved, then add 17.8g (114mmol) dicyclohexylcarbodiimide Amine was reacted at room temperature for 4 hours. After the reaction, solid-liquid separation was carried out to obtain a tetrahydrofuran solution of Ac-D-Lys(Boc)-COOSu.
[0039](3) Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0040] Add 180mL of a mixed aqueous solution containing 1...
Embodiment 2
[0055] 1. Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0056] (1) Synthesis of Ac-D-Lys(Boc)-OH
[0057] Mix and dissolve 24.6g (100mmol) H-D-Lys(Boc)-OH with 200mL dichloromethane and 12.1mL (70mmol) N,N'-diisopropylethylamine, then add 12.3mL (130mmol) acetic anhydride, room temperature The reaction was carried out for 2 hours; after the reaction solution was concentrated, diethyl ether was added to make a slurry, filtered, the filter cake was washed, and vacuum-dried at room temperature to obtain 17.5 g of Ac-D-Lys(Boc)-OH.
[0058] (2) Synthesis of Ac-D-Lys(Boc)-COOSu
[0059] Mix 17.5g (93mmol) Ac-D-Lys(Boc)-OH, 200mL tetrahydrofuran and 12.8g (112mmol) N-hydroxysuccinimide until dissolved, then add 18.8g (121mmol) dicyclohexylcarbodiimide Amine was reacted at room temperature for 4 hours. After the reaction, solid-liquid separation was performed to obtain a tetrahydrofuran solution of Ac-D-Lys(Boc)-COOSu.
[0060] (3) Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0061] Add...
Embodiment 3
[0076] 1. Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0077] (1) Synthesis of Ac-D-Lys(Boc)-OH
[0078] Mix and dissolve 24.6g (100mmol) H-D-Lys(Boc)-OH with 200mL dichloromethane and 13.8mL (80mmol) N,N'-diisopropylethylamine, then add 14.2mL (150mmol) acetic anhydride, room temperature The reaction was carried out for 1.5 hours; after the reaction solution was concentrated, diethyl ether was added to make a slurry, filtered, the filter cake was washed, and vacuum-dried at room temperature to obtain 17.3 g of Ac-D-Lys(Boc)-OH.
[0079] (2) Synthesis of Ac-D-Lys(Boc)-COOSu
[0080] Mix 17.3g (92mmol) Ac-D-Lys(Boc)-OH, 200mL tetrahydrofuran and 13.8g (120mmol) N-hydroxysuccinimide until dissolved, then add 20.1g (129mmol) dicyclohexylcarbodiimide Amine was reacted at room temperature for 6 hours. After the reaction, solid-liquid separation was carried out to obtain a tetrahydrofuran solution of Ac-D-Lys(Boc)-COOSu.
[0081] (3) Synthesis of Ac-D-Lys(Boc)-Asp(OtBu)-OH
[0082]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com